SERVICES
Wearables
What are Wearable Devices?
Wearable devices in clinical trials and digital health can provide patient monitoring, surveillance, screening, diagnosis and assistance with treatment, post-treatment and on-going management. These devices also determine, and confirm, efficiency of treatment based on real-time physiological feedback.

Improving Clinical Trials Through Wearables
This has led to clinical sites’ investigation of and subsequent implementation of wearable technology in clinical trials. Currently, about 10% to 15% of trials are incorporating wearable devices, primarily to collect data as exploratory endpoints (PharmaVoice); and, according to research by Berg Insights, the number of remotely monitored patients is expected to reach 50.2 million by this year.
Explore the Endless Possibilities
of Our Wearable Devices

Unparalleled Data
Collection
Patient-Centric
Approach
Our technology platform also provides diverse and underrepresented patient populations access to quality healthcare and clinical trials—closing the inequality gap created by social determinants of health (SDOH)—removing barriers and increasing access to all patients worldwide.


Raw Data Capture (RDC)
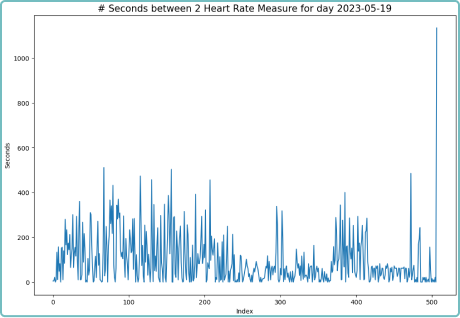
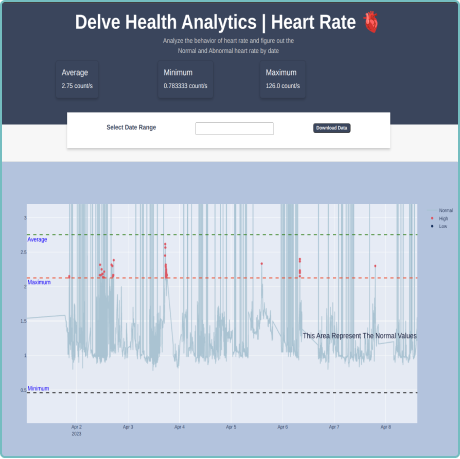
We understand the importance of raw data in clinical trials. Our configurable platform captures and stores raw data, providing researchers with a wealth of detailed information for in-depth analysis. This raw data capture allows for a comprehensive understanding of patients’ physiological parameters and behaviors, enabling researchers to utilize and develop their own algorithms against the raw data collected. This benefit supports researchers’ efforts and provides them with the opportunity to uncover hidden patterns and correlations. With access to unfiltered data, you can extract valuable insights, drive scientific breakthroughs and make data-driven decisions with confidence. By embracing raw data capture, Delve Health empowers you to explore the full potential of wearable devices in clinical trials, all from within a powerful, single analytical application—driving ease of use, better full data sets, quicker results, better outcomes and ultimately safely speeding-up the timelines to offering medications and therapies in the marketplace for patients.
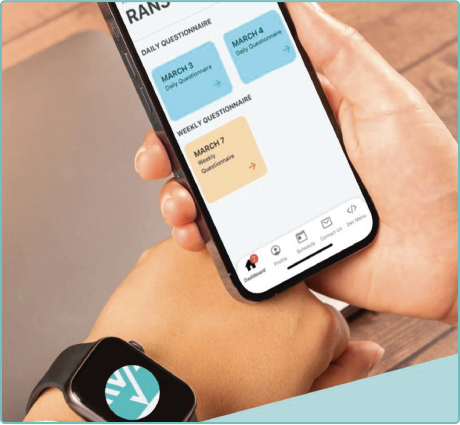
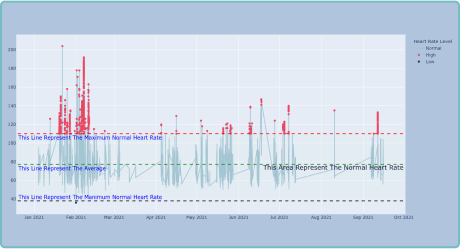
Real-Time Monitoring
With real-world data (RWD) in real-time streaming, Clinical StudyPal empowers researchers to monitor patients’ progress remotely. This eliminates the need for frequent site visits and enables prompt interventions when necessary. By closely tracking patients’ health parameters, we optimize trial outcomes and enhance patient safety.


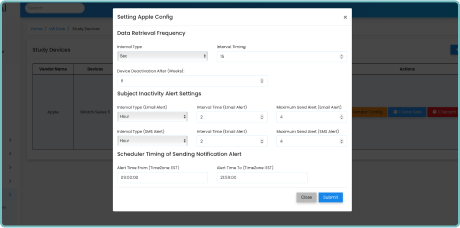

Seamless Integration
Integrating with existing clinical trial platforms is effortless with Delve Health’s solution. Our wearable devices seamlessly synchronize with your preferred data management systems, ensuring streamlined workflow. We provide secure, encrypted data transmission, protecting patient privacy, as we are HIPPA, GDPR, and CFRpart11 compliant, while facilitating efficient data analysis.
Empowering Research Insights
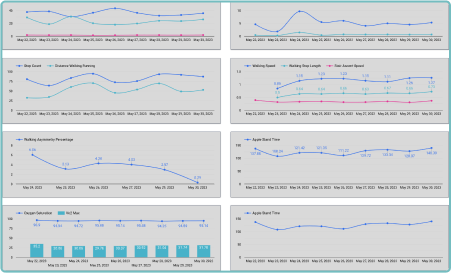


Enhanced Trial Efficiency
By leveraging wearable devices and biosensors in clinical trials, Delve Health significantly improves trial efficiency. Our innovative technology reduces data collection time, minimizes errors and accelerates the analysis process. Researchers gain deeper insights faster, enabling quicker decision-making and facilitating the development of life-changing therapies.
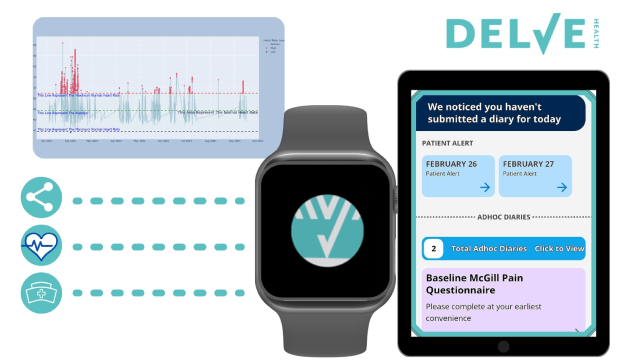

Are You Looking to Transform the Way Clinical Research is Conducted?
Delve Health’s hybrid model of BYOD and fully-configured, agnostic devices has enabled patients, clinicians and caregivers to report outcomes for more granular endpoint data—utilizing more sensitive measures than traditional clinical scales; our wearables program makes it easier for your team to conduct extended remote studies and easier for patients to engage.