Lean operations
Fewer vendors
One platform + one team
Study execution
Faster
Configured and deployed in days
Data quality
Cleaner
Real-time QC + human follow-through
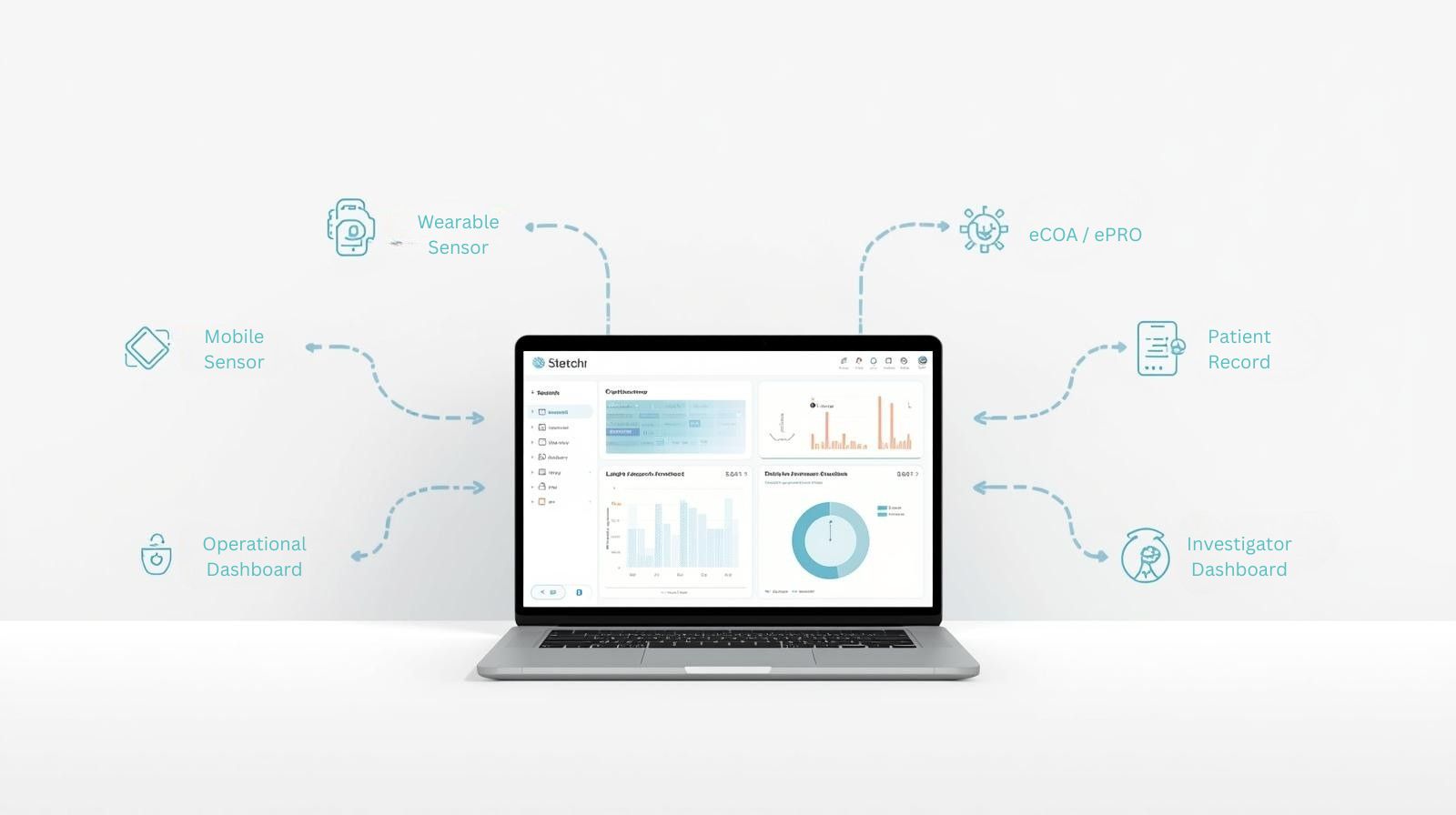
Unified Study Execution
Build → Deploy → Monitor → Resolve
Your Study Needs Speed — Not Complexity
Biotechs need to move quickly, operate lean, and build evidence that stands up to regulatory scrutiny. Delve Health brings together eCOA, wearables, diaries, patient engagement, and concierge support into one platform purpose-built for smaller teams.
- Fit-for-purpose eCOA + wearable platform
- Reduced operational burden for small teams
- Adaptive workflows for early-phase trials
- Faster deployment with minimal IT overhead
Built for Emerging Biotechs
Move fast without sacrificing data quality. Delve gives lean teams a unified platform for eCOA, wearables, and compliance—backed by real humans when execution gets messy.
Fast Deployment
Launch eCOA and wearables in weeks, not months.
Fit-for-Purpose Endpoints
Validated digital biomarkers aligned with regulatory expectations.
Human Concierge
Multilingual outreach keeps participants engaged and compliant.
Lean Team Ready
Automated oversight plus human support reduces operational load.
eCOA + Wearables in One Place
Early-phase and exploratory studies rely on accurate, continuous data. Delve provides a single, validated infrastructure for collecting both subjective and objective endpoints.
- Wearable endpoints aligned with FDA/EMA frameworks
- ePRO + symptom scoring with audit trails
- Real-time device and diary compliance monitoring
- Objective–subjective signal correlation
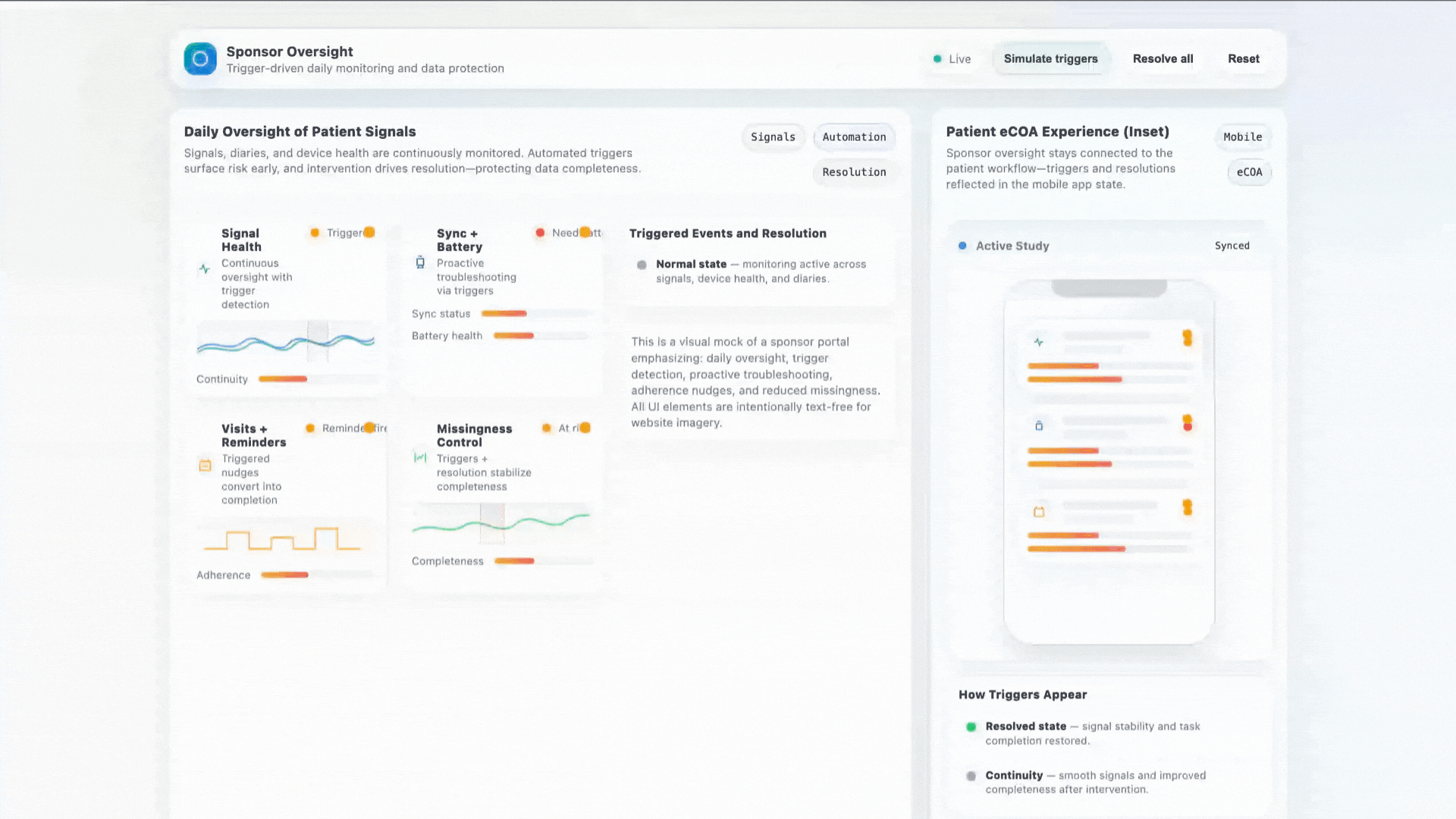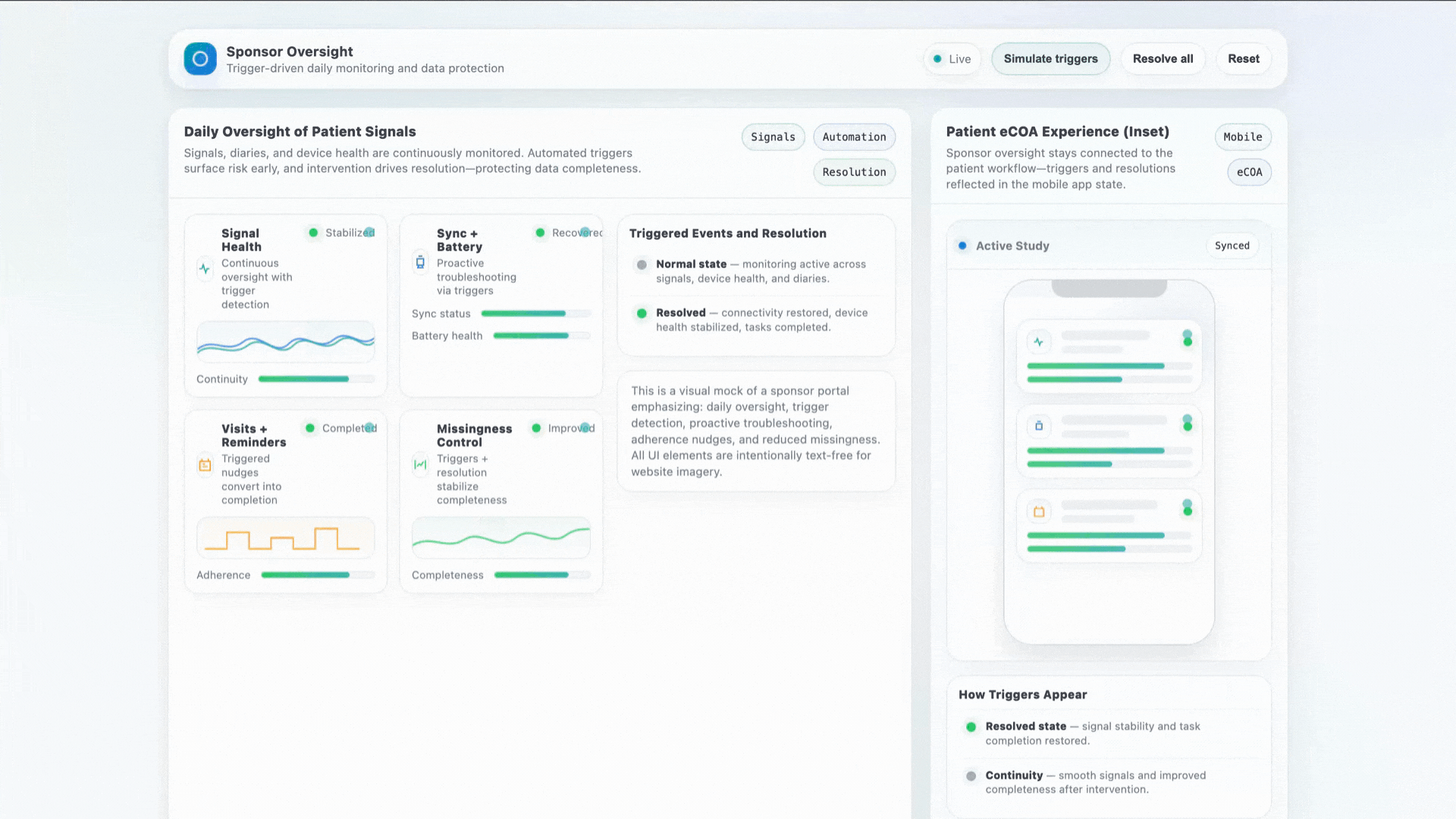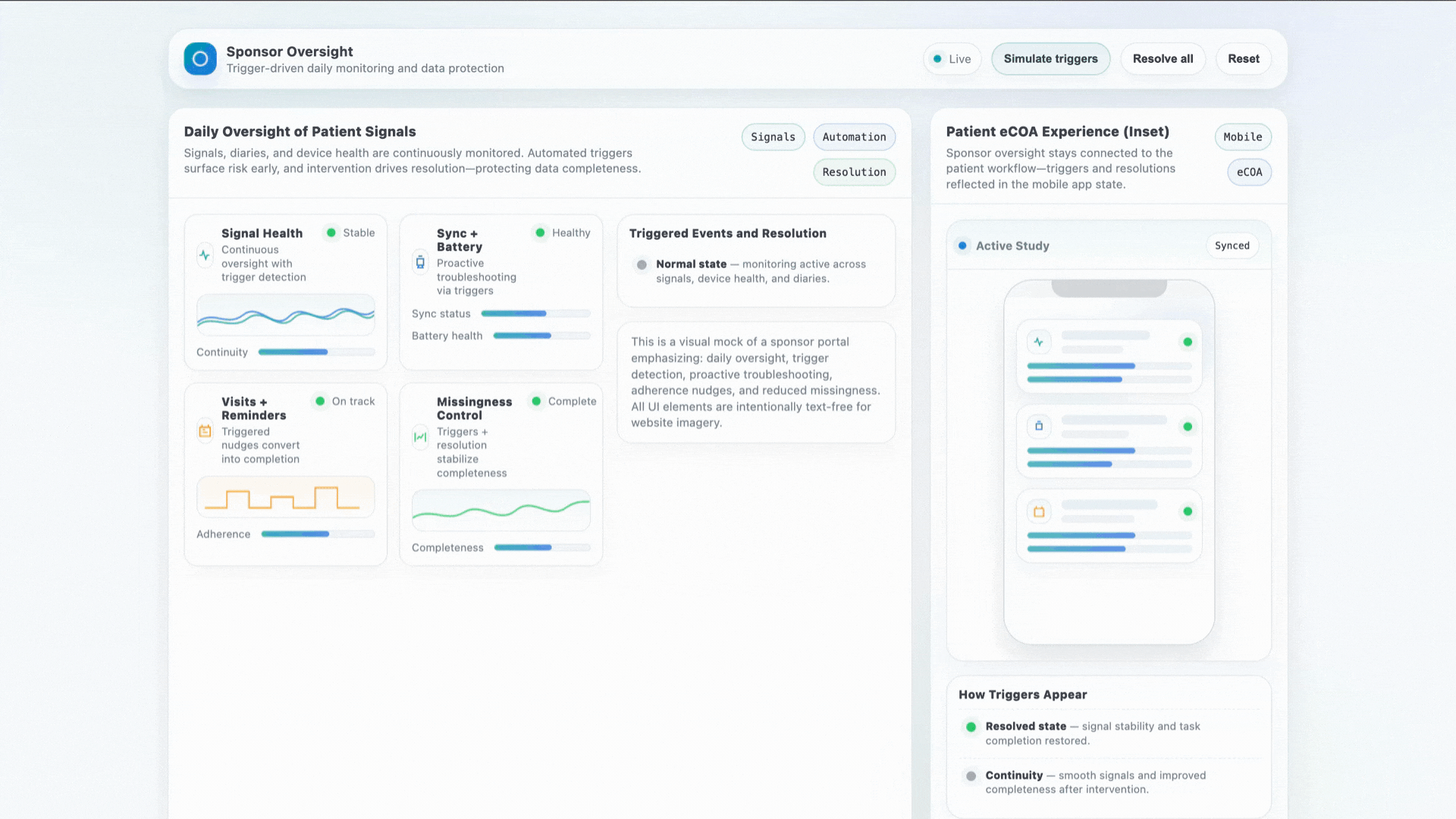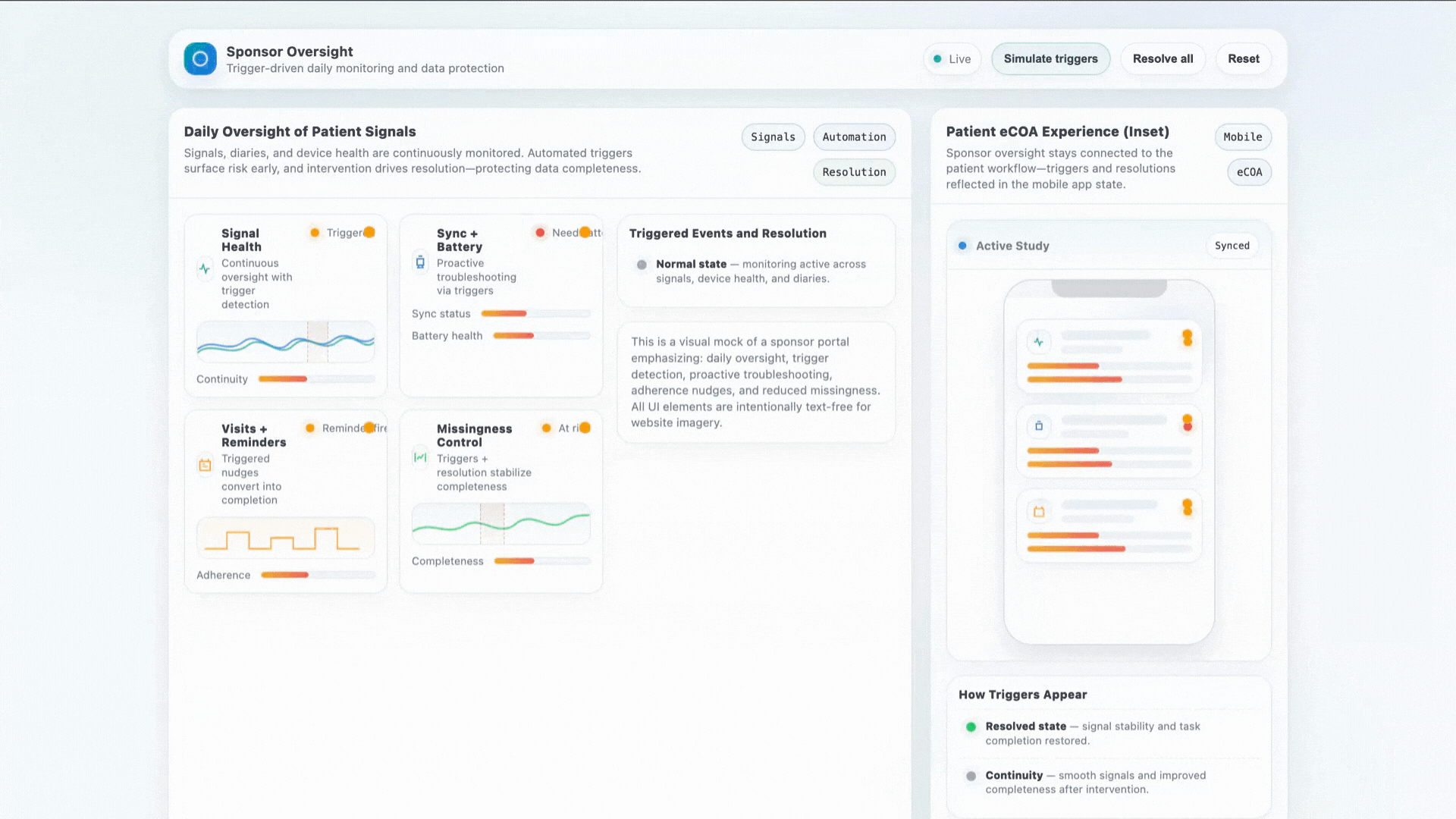
Stronger Early Data With Human Support
Early-phase studies struggle with variability. When a patient gets stuck, a device misbehaves, or a diary is missed — our concierge intervenes in real time to protect data quality.
- Daily oversight of patient signals
- Proactive sync + battery troubleshooting
- Visit reminders and adherence coaching
- Significant reduction in missingness
Powering Evidence for Emerging Biotechs
Early-Phase Digital Endpoint Study
Objective signals validated against gold-standard methods.
Decentralized Rare Disease Trial
92% ePRO completion across a hard-to-reach population.
Exploratory Biomarker Program
35% faster data readouts with real-time dashboarding.
Move Your Science Forward — Faster.
One platform. Validated endpoints. Human retention. Everything a biotech needs to scale evidence.
Book a Biotech Demo