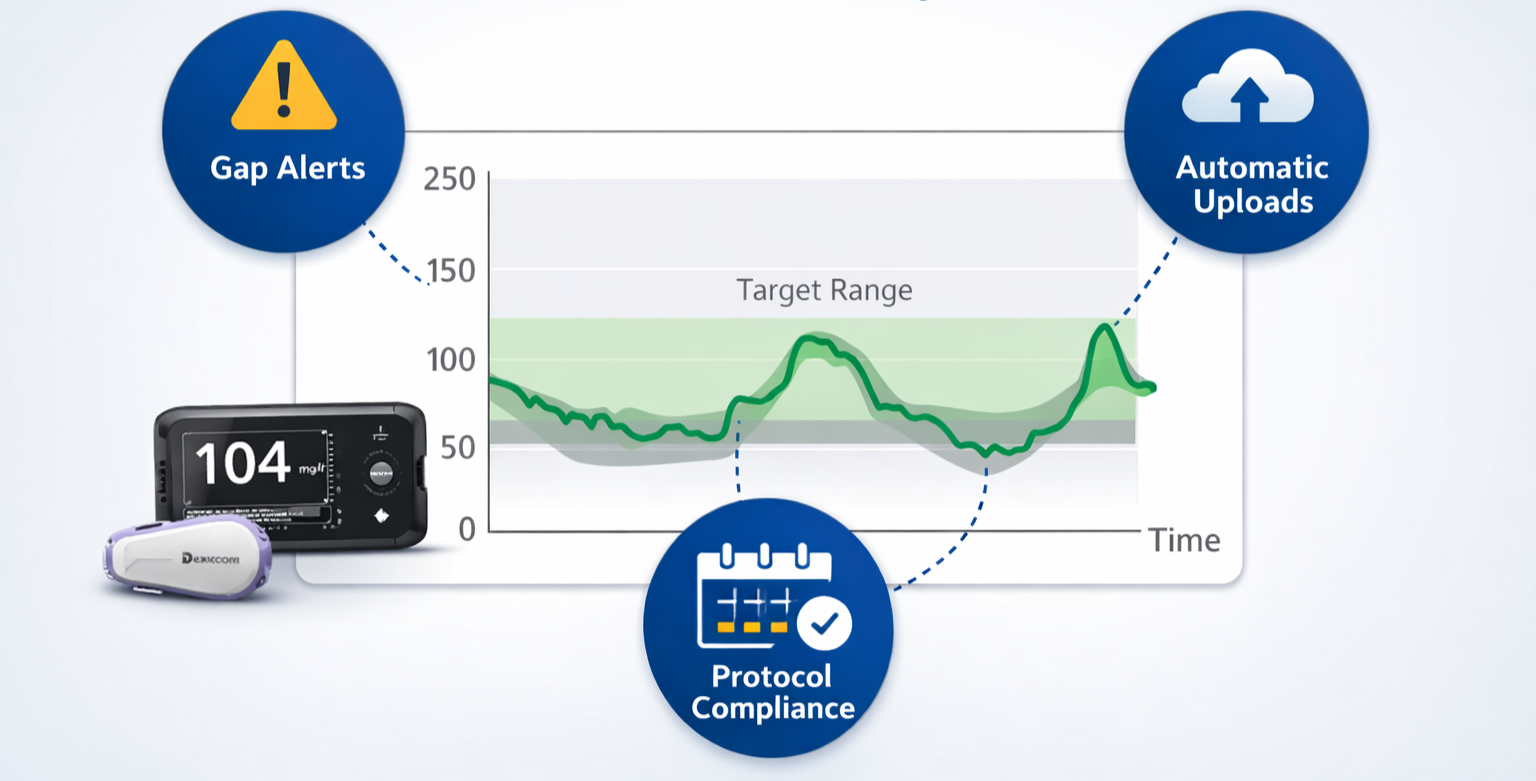
CGM continuity (Dexcom)
Continuous glucose capture with monitoring for gaps, uploads, and protocol-defined windows—so outcomes remain interpretable.
Diabetes, Obesity & GLP-1 Programs
Missed CGM uploads. Weigh-ins that don’t happen. Adherence drift between visits. Delve combines Dexcom CGM, Withings digital weight capture, and activity wearables with a real-time compliance operating model and concierge follow-through—so you get usable endpoints, not just collected data.
Built for metabolic realities
In metabolic programs, the endpoint integrity depends on consistent daily capture: CGM continuity, weigh-in routines, and activity adherence. Delve monitors signal health, rescues adherence drift, and documents interventions—before missingness becomes the headline.

Continuous glucose capture with monitoring for gaps, uploads, and protocol-defined windows—so outcomes remain interpretable.
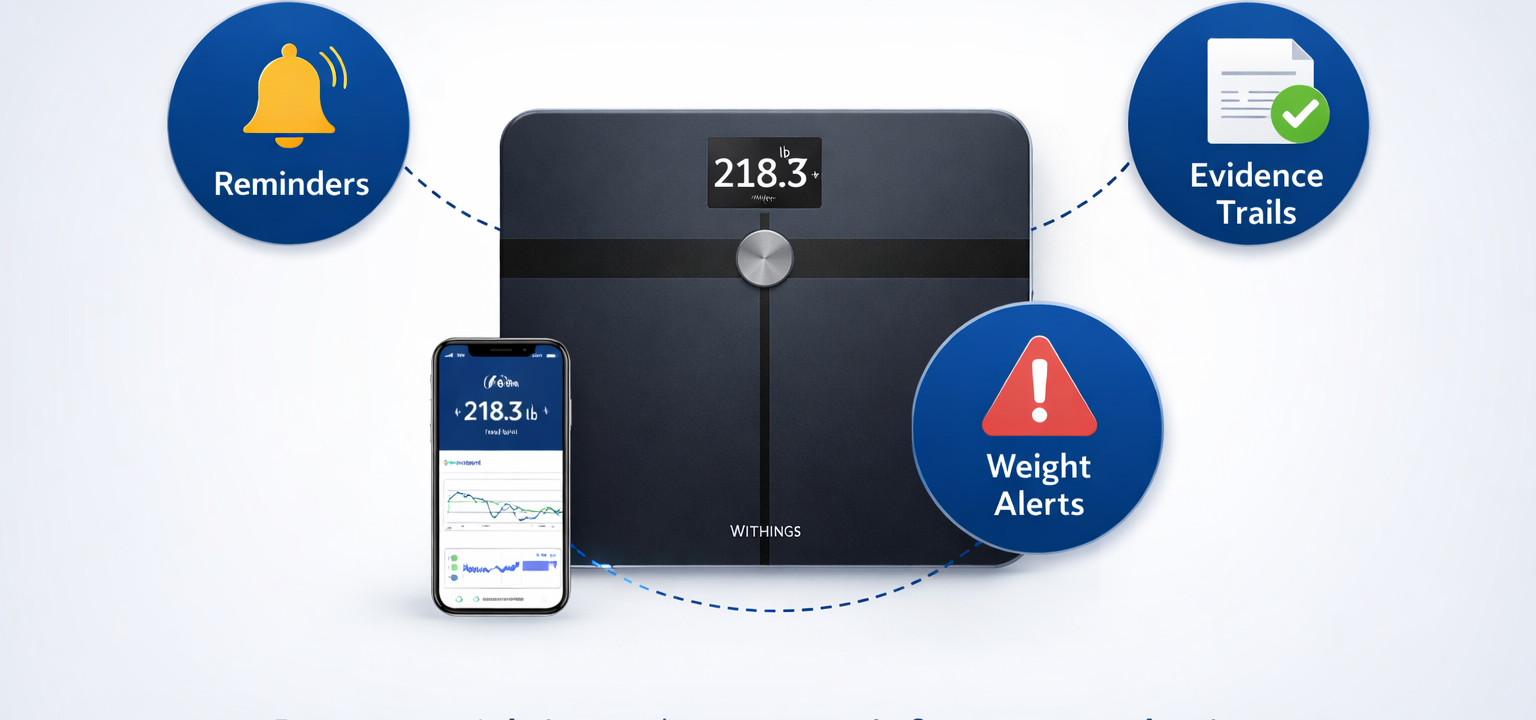
Remote weigh-ins with routine reinforcement, alerting, and evidence trails—reducing site follow-up burden.
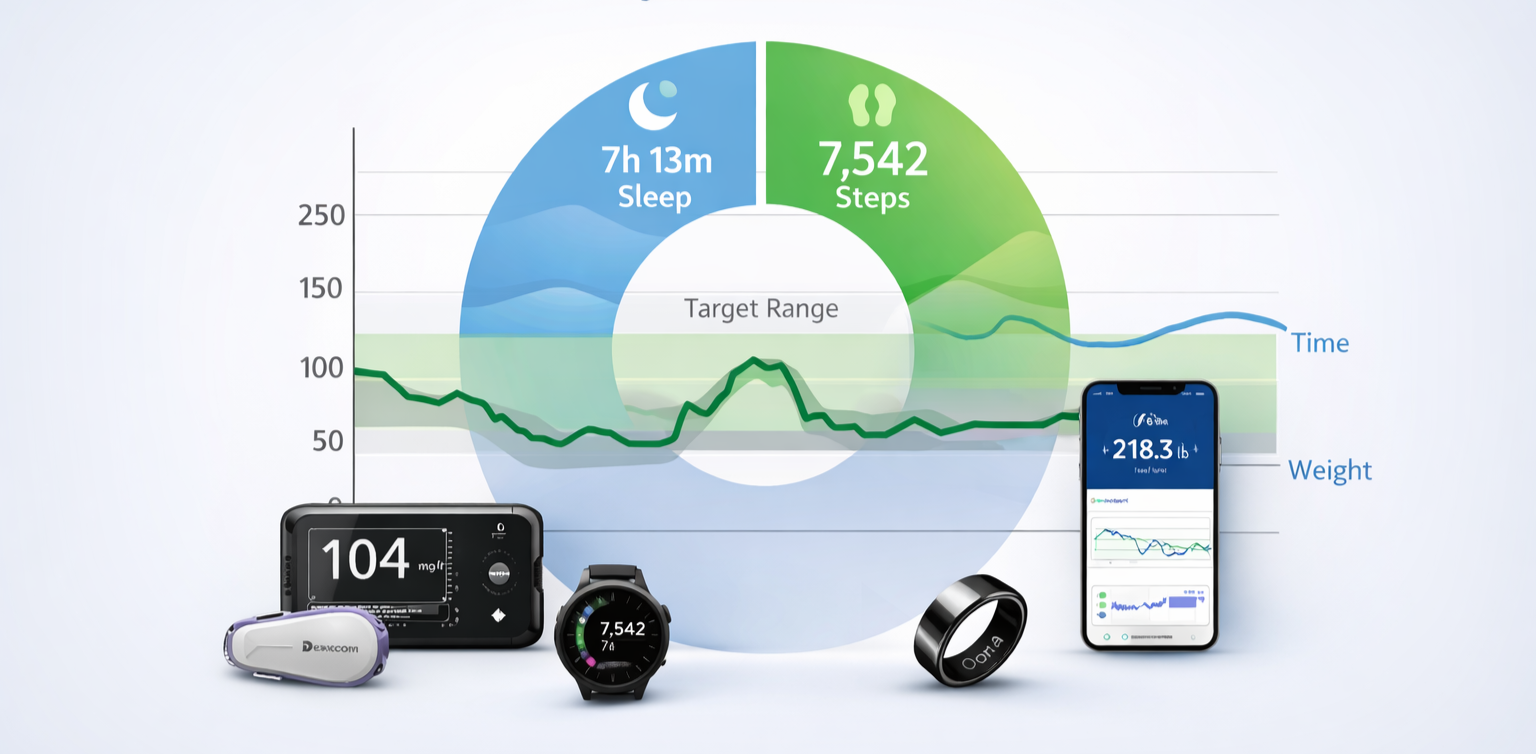
Wearables provide activity/sleep context for weight and glucose patterns—captured continuously, QC’d, and export-ready.
Why Delve
Diabetes, obesity, and GLP-1 endpoints degrade through day-to-day friction: CGM gaps, skipped weigh-ins, scale issues, app fatigue, and routine breakdowns. Delve closes these gaps by owning adherence operations and device telemetry—continuously, not retrospectively.
Risk: CGM and weigh-in drift accumulates quietly; missingness becomes irreversible inside protocol windows.
Risk: the dataset locks with preventable gaps and post-hoc cleaning becomes the workflow.
Outcome: higher usable data yield, fewer deviations, lower site burden, stronger retention.
Metabolic Evidence Chain
Metabolic endpoints are sensitive to missingness and adherence. Delve closes the loop with telemetry, quality gates, and documented interventions when the chain breaks.
How We Engage
Delve leads execution end-to-end with a metabolic operating model built around continuous capture, adherence rescue, and protocol-defined interventions. Specialty functions can be delivered directly or through Delve-led partners as scoped.
Keep your CRO for monitoring/EDC and plug Delve in as the digital capture + adherence operations layer. We reduce missingness, improve device adherence, and provide live risk visibility.
We offload the most time-consuming follow-up work—device troubleshooting, weigh-in outreach, reminders, and re-training—so sites can focus on enrollment and clinical oversight.
Live Metrics (Example)
These are example visuals to demonstrate how we monitor CGM continuity, weigh-in adherence, and wearable sync in real time. Your study’s dashboards reflect your protocol, schedules, and site structure.
Markers indicate documented interventions (coaching, troubleshooting, replacements) that prevent gaps.
Routine reinforcement and escalation reduce missed weigh-ins and support retention.
Telemetry surfaces connectivity issues early so the team can recover data within protocol windows.
Protocol-to-Analysis
“End-to-end” means ownership with evidence trails—from protocol operationalization and device plans through compliance rescue, data QC, and analysis readiness.
Step 1
Step 2
Step 3
Step 4
Step 5
Step 6
Step 7
Step 8
Metabolic Capabilities
Metabolic endpoints require continuity. We monitor capture, gaps, and adherence so glucose data is usable.
Weight change is only meaningful if weigh-ins are consistent. We build and enforce the routine.
Activity and sleep explain variance in glucose and weight. We collect, QC, and reconcile these streams.
Deliverables
Metabolic trials don’t fail because the endpoint is unclear. They fail because the routine breaks. Delve operationalizes CGM continuity, weigh-in adherence, and wearable capture—then documents interventions—so your datasets are usable.
If you already have DM/biostats partners, Delve strengthens the operational evidence chain without replacing your stack.
If your endpoints depend on consistent daily capture—CGM continuity, remote weigh-ins, activity context—Delve is built to protect that evidence chain. We can lead end-to-end execution or integrate with your CRO to improve compliance, retention, and usable data yield.