
Photo QC & re-capture loops
Quality gates for lighting, framing, and consistency—plus re-capture prompts before unusable images spread.
Dermatology Trials
Delve runs the between-visit operating model: topical routines, symptom capture, and standardized photography—backed by concierge follow-through and live oversight—so missingness gets resolved early, not explained later.
Dermatology studies live and die between visits: topical adherence, flare timing, itch and pain capture, standardized photography, and consistent assessment routines. Delve combines CRO-style execution with a real-time compliance operating model—eCOA/ePRO, photo workflows, concierge follow-through, kit logistics, and live oversight— so you get usable evidence, not just collected data.
Between-visit adherence
Owned
Topicals, diaries, photo cadence
Data quality
Live
Photo QC + missingness alerts
Site burden
Lower
Concierge absorbs follow-up load
Explore Delve by therapeutic area
Dermatology is one view of the same operating system: compliance ownership, reduced site burden, and real-time intervention. Explore other therapeutic areas — and the post-market hub that ties them together.
High-burden protocols where between-visit symptom capture and retention decide data integrity.
Explore oncology execution →
Small cohorts where every missed day matters — caregiver workflows, logistics, and adherence rescue.
Explore rare disease execution →
CGM + weight + long follow-up where device fatigue and silent dropout must be detected early.
Explore metabolic execution →
Fatigue-sensitive tasks and wear-time risk — adherence operations matters more than dashboards.
Explore CNS execution →
The operating model for 3–5 year follow-up: detect drift early, intervene fast, and keep endpoints usable.
Go to post-market hub →
Long-term physiological monitoring where wear-time decay, silent data loss, and delayed escalation threaten endpoint integrity.
Explore cardiology execution →
Designed for dermatology realities
Dermatology success depends on consistency between visits. Delve enforces imaging standards, supports adherence routines, and rescues missingness before it becomes a protocol deviation.

Quality gates for lighting, framing, and consistency—plus re-capture prompts before unusable images spread.

Topical routines, diaries, and symptom capture reinforced by reminders and human follow-through.

Concierge handles participant training, troubleshooting, and rescheduling—so coordinators can focus on assessments.
Why Delve
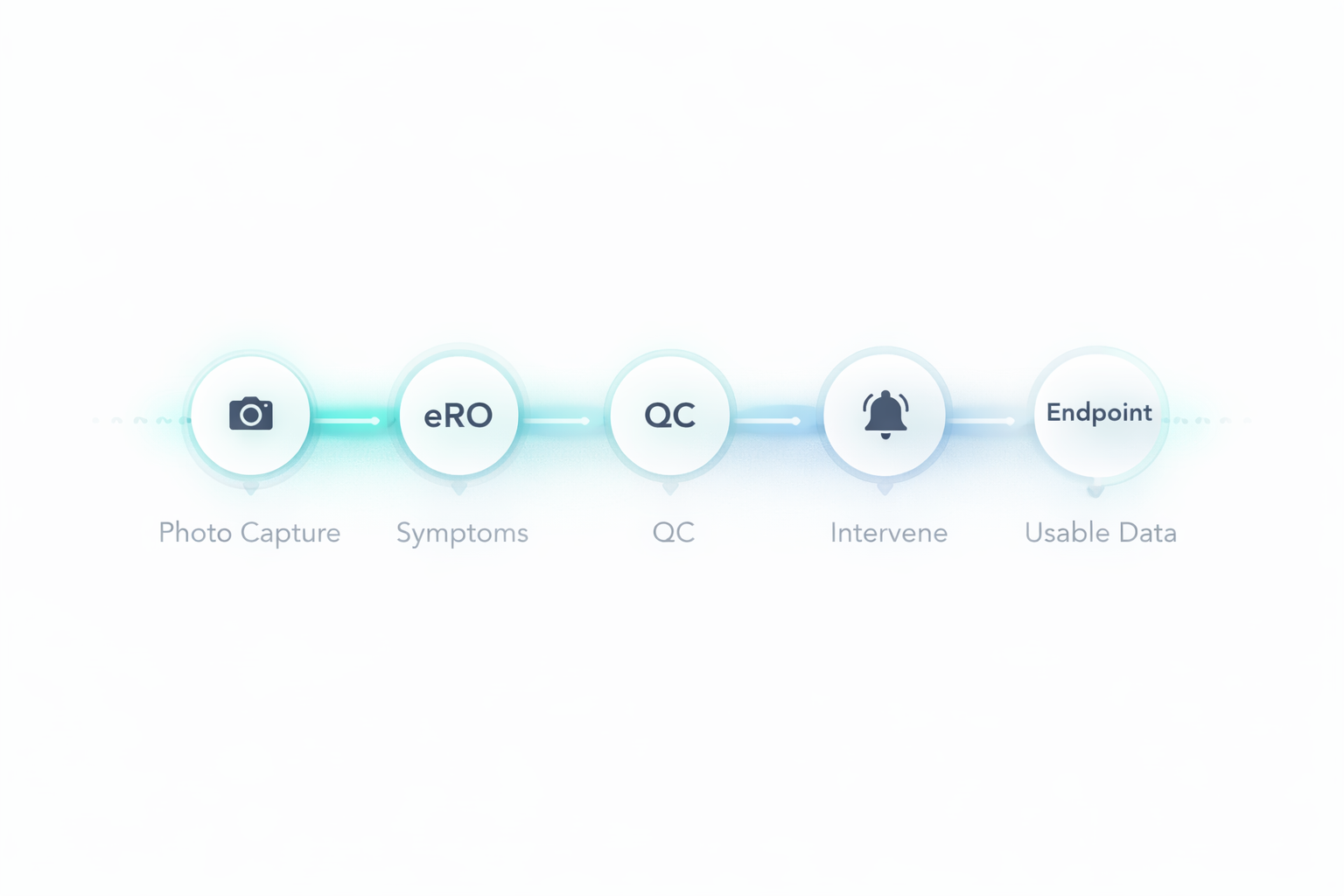
In dermatology, the evidence chain breaks through routine friction: topicals are skipped, photos are inconsistent, symptoms go unreported, and flare timing is missed. Delve closes these gaps by owning adherence operations and documenting interventions—continuously, not retrospectively.
Risk: derm adherence and photo quality degrade between visits; issues are discovered late and fixed expensively.
Risk: “missingness” becomes the story, not the endpoint.
Outcome: higher usable data yield, fewer protocol deviations, lower site burden, stronger retention.
Dermatology Evidence Chain
Dermatology endpoints are especially sensitive to between-visit variability. Delve closes the loop with quality gates, real-time alerts, and documented interventions when the chain breaks.
How We Engage
Delve leads execution end-to-end with a derm operating model built around adherence, imaging quality, and real-time oversight. Specialty functions can be delivered directly or through Delve-led partners as scoped.
Keep your CRO for monitoring/EDC and plug Delve in as the compliance, imaging, and participant operations layer. We reduce missingness, improve photo consistency, and provide live risk visibility.
We offload the most time-consuming derm follow-up work—topical reminders, diary completion, photo coaching, and re-capture—so sites can focus on assessments and enrollment.
Live Metrics (Example)
These are example visuals to demonstrate how we monitor adherence, imaging quality, and completion trends in real time. Your study’s dashboards reflect your protocol, schedules, and site structure.
Markers indicate documented interventions (coaching, re-training, rescheduling) that prevent drift.
Quality gates surface low-confidence images early, enabling re-capture within protocol windows.
Sites with higher friction get proactive concierge support and targeted retraining workflows.
Protocol-to-Analysis
“End-to-end” means ownership with evidence trails—from protocol operationalization and imaging standards through compliance rescue, data QC, and analysis readiness.
Step 1
Step 2
Step 3
Step 4
Step 5
Step 6
Step 7
Step 8
Dermatology Capabilities
Derm efficacy signals are sensitive to dosing routines, washout rules, and daily behavior—not just site visits.
Low-quality at-home photos silently corrupt endpoints. We enforce capture standards before data is lost.
Itch, pain, sleep disruption, and flare timing need consistent capture—without adding workload to sites.
Deliverables
Derm studies don’t fail because the endpoint is unclear. They fail because the routine breaks. Delve operationalizes adherence, enforces imaging standards, and documents interventions—so your datasets are usable.
If you already have DM/biostats partners, Delve strengthens the operational evidence chain without replacing your stack.
Download
A practical operating model for long-term follow-up: how to detect compliance drift early, intervene fast, reduce site burden, and keep endpoints usable across eCOA + photos + wearables.
If your endpoints depend on consistent behavior between visits—topicals, diaries, photos, flare reporting—Delve is built to protect that evidence chain. We can lead end-to-end execution or integrate with your CRO to improve compliance and retention.
FAQ
The failure points that quietly break derm endpoints: photos, symptoms, adherence, and between-visit drift.
We enforce capture standards (lighting, framing, distance, angle, reference markers) and use quality gates plus re-capture loops so unusable photos are corrected inside protocol windows—not discovered at the visit or at lock.
Derm endpoints depend on daily routines: topical adherence, symptom capture, and photo cadence. Drift starts small and compounds. Without an operating model that intervenes early, missingness becomes normalized.
eCOA can capture tasks and generate alerts, but it doesn’t guarantee recovery. Delve pairs real-time monitoring with concierge follow-through to resolve missed entries, confusion, and device friction in hours—not weeks.