Wearable devices are transforming how clinical trials are conducted across the globe. From improving patient engagement to collecting continuous, real-time health data, these tools are enhancing both efficiency and accuracy in research. In modern clinical studies, wearable technology has become a key factor in ensuring better outcomes, smoother operations, and more reliable data collection.
For researchers and Contract Research Organizations (CROs), the integration of wearable devices represents a significant shift toward smarter, more connected healthcare solutions.
Understanding Wearable Devices in Clinical Research
Wearable devices are compact, sensor-equipped technologies that track physiological parameters such as heart rate, temperature, movement, or oxygen levels. These devices can be worn on the wrist, chest, or other body parts, continuously collecting data that feeds directly into clinical databases.
By eliminating the need for frequent in-person checkups, wearables help reduce patient burden while ensuring constant data flow for researchers.
How Wearable Devices Work
Wearables transmit data through wireless connections, often synced with secure cloud platforms. They provide near real-time monitoring, helping investigators detect health patterns, measure adherence, and track treatment responses.
Why They’re Gaining Popularity
Researchers value wearable technology for its accuracy, convenience, and ability to engage participants outside clinical settings. It supports decentralized trials, allowing for broader participation across different locations.
Enhancing Participant Monitoring
Continuous participant monitoring is one of the most significant advantages of using wearable devices in clinical trials.
Real-Time Data Collection
Instead of relying on periodic assessments, wearables capture physiological data continuously. This approach gives researchers access to detailed health patterns that help identify early changes in patient conditions.
Early Detection of Adverse Events
Instant alerts from wearable devices allow investigators to address potential safety concerns promptly. This proactive response helps ensure patient safety and regulatory compliance.
Improving Data Accuracy and Consistency
Data accuracy is essential in clinical research, and wearable devices have revolutionized this aspect.
Reducing Human Error
Traditional manual reporting often leads to inaccuracies or missed entries. With digital data capture, the margin for human error decreases significantly, ensuring high-quality and reliable results.
Standardized Data Capture
Wearables collect structured, timestamped data across all participants, creating consistent datasets that are easier to analyze and validate.
Enabling Remote and Decentralized Trials
The rise of decentralized trials has been accelerated by wearable technology. Participants can now contribute valuable health data from the comfort of their homes.
Convenience for Participants
By removing the need for frequent clinic visits, wearables reduce travel-related stress and time constraints. This flexibility makes participation easier and improves overall satisfaction.
Wider Participant Inclusion
Decentralized approaches supported by wearable devices allow researchers to recruit participants from different regions and demographics, creating more diverse and representative studies.
Compliance and Automation
Ensuring participant compliance is one of the toughest challenges in clinical trials. Wearables help solve this through automation and continuous monitoring.
Tracking Adherence
Many wearable devices are equipped with sensors that monitor medication timing or activity levels, ensuring participants follow study protocols accurately.
Automating Data Transmission
Automated uploads eliminate delays in reporting, providing real-time updates to clinical teams and reducing administrative work.
Enhancing Patient Engagement and Experience
Engaged participants are more likely to stay in a study until completion. Wearable devices foster engagement through feedback, motivation, and personalized tracking.
Empowering Participants
Wearables provide users with real-time health information, allowing them to understand how their participation contributes to the research. This sense of involvement increases retention.
Boosting Motivation Through Feedback
Visual dashboards and progress reports help participants stay motivated by showing them the impact of their efforts on study outcomes.
Streamlining Data Analysis for Researchers
Data from wearable devices simplifies and strengthens the analytical process.
Continuous Data Streams
Wearables generate large volumes of time-stamped data, giving researchers a comprehensive view of patient health over time.
Improving Decision-Making
Machine learning algorithms and analytics platforms can process wearable data to identify patterns, predict outcomes, and guide clinical decisions faster than traditional methods.
Facilitating Regulatory Compliance
Regulatory bodies emphasize accuracy, transparency, and participant safety in clinical research. Wearables assist in maintaining these standards effectively.
Audit-Ready Data
Automatically collected and securely stored data reduces documentation errors, ensuring compliance with data integrity regulations.
Transparent Data Trails
Digital records from wearables provide a traceable history of participant activity, supporting audits and regulatory submissions.
Optimizing Trial Efficiency
Technology-driven efficiency helps research teams conduct trials faster and more effectively.
Reducing Operational Delays
Real-time data reduces waiting times between participant visits, allowing quicker insights and decision-making.
Lowering Overall Costs
Fewer in-person visits and streamlined data processing can help reduce operational expenses without compromising quality.
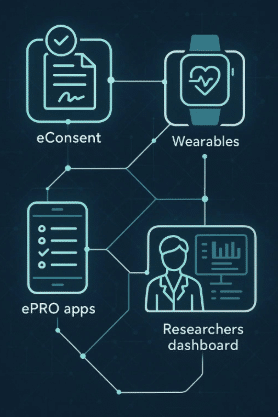
Integration with Digital Platforms
Seamless integration of wearables with digital health platforms makes clinical operations more connected.
Cloud-Based Data Storage
Cloud platforms store and process data securely, enabling real-time access for multiple stakeholders, from research teams to sponsors.
Interoperability with Other Systems
Wearables can connect with electronic data capture (EDC) and clinical trial management systems (CTMS), ensuring smooth workflow integration.
Building Participant Trust Through Transparency
Trust is essential in maintaining long-term participant engagement.
Privacy and Data Security
Most wearable technologies use encryption and secure data channels to protect sensitive participant information.
Participant Confidence
When participants know their data is secure and used ethically, they are more likely to stay engaged and compliant throughout the study.
Promoting Continuous Innovation in Clinical Research
Wearable devices are driving continuous innovation in trial methodologies.
Advancing Precision Medicine
Real-time data from wearables supports the development of personalized treatments and adaptive trial designs.
Encouraging Data-Driven Decisions
The ability to collect longitudinal data helps researchers make evidence-based adjustments during the trial process.
Conclusion
Wearable devices are redefining how clinical trials are conducted, providing measurable improvements in accuracy, compliance, patient experience, and patient retention in clinical trials. They enable continuous monitoring, reduce human error, and foster participant engagement, all of which lead to more reliable and efficient outcomes.
For researchers and CROs, adopting wearable technology is not just a trend it is a strategic move toward a more connected, patient-centered, and data-driven future of clinical research.
FAQs
How do wearable devices benefit clinical trials?
They provide real-time health data, reduce manual reporting, and enhance patient engagement and compliance.
Are wearable devices secure for patient data?
Yes. Most devices follow strict data protection protocols with encryption and secure cloud storage.
Can wearables replace traditional clinical visits?
Not entirely, but they significantly reduce the need for frequent visits by allowing remote monitoring.
What kind of data do wearables collect in trials?
They collect physiological data such as heart rate, movement, sleep, temperature, and other relevant health metrics.
How do wearable devices improve participant retention?
By making participation convenient and interactive, they reduce burden and keep patients more engaged throughout the study.