SERVICES
Patient eConsent Clinical Trials
Clinical StudyPal’s eConsent service automates the patient enrollment process, on-boarding patients more efficiently—securely and ethically.
BENEFITS OF CLINICAL TRIAL eCONSENT INCLUDE, BUT ARE NOT LIMITED TO
eConsent is enhancing the way clinical trials are conducted by providing a digital, user-friendly method for obtaining informed consent from patients. This transformation offers numerous benefits, including:
- Efficient Use of Time via Automation: Automation accelerates the study start-up process, allowing for quicker patient enrollment and reducing overall costs.
- Reduction in Informed Consent Errors: By automating the consent process, eConsent minimizes the risk of errors, ensuring that patients fully understand the trial requirements.
Remote Consent Monitoring: With the ability to monitor consent remotely, eConsent facilitates the participation of patients from any location, enhancing the reach of your clinical trial.
Enhanced Patient Experience: A seamless and intuitive interface increases patient compliance and retention by providing a smooth onboarding process.
Guaranteed Signature Compliance: eConsent ensures that all signatures are collected correctly, whether through hand signatures or video consent.
Advanced Security Features: Our platform uses facial recognition technology to enhance patient privacy and security, safeguarding sensitive information.

HOW CAN WE IMPLEMENT eCONSENT SOFTWARE CLINICAL TRIALS FOR OUR STUDY?
Learn more about eConsent and integrating Delve Health’s Clinical StudyPal platform, in order to design hybrid or decentralized clinical trials, by requesting a demonstration.
eConsent can lead to a transformative experience for study participants by providing simple, easy-to-comprehend clinical trial information—boostING patient engagement and improving the patient experience overall.
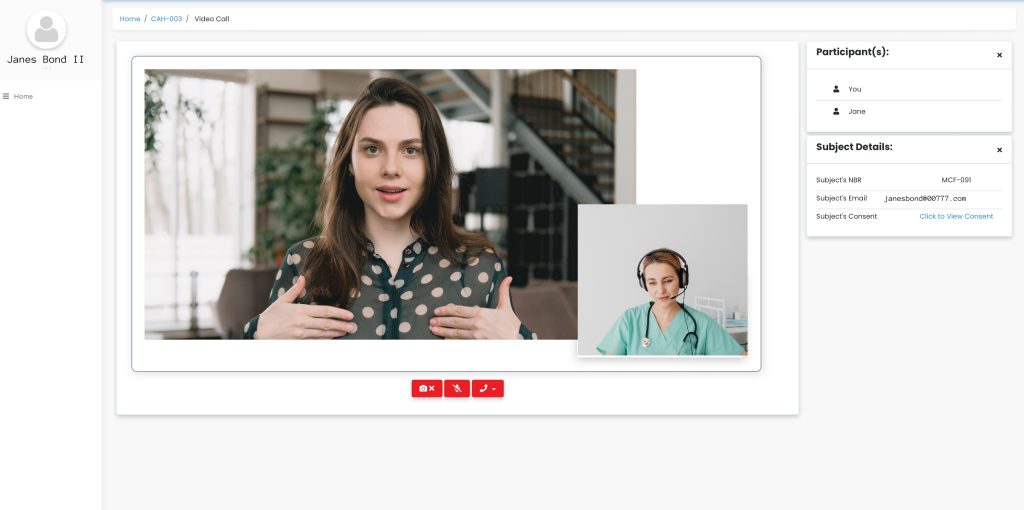
Enable video calls, provide interactivity and support the flexibility offered to patients by eConsent.
Delve Health’s Clinical StudyPal platform is web-based, offering complete a configurable eConsent solution for every study.
Customizable and Interactive
Our eConsent software clinical trials are fully customizable to meet the specific needs of your study. You can enhance the consent process by adding multimedia elements, such as videos, links to frequently asked questions, and other interactive components. This customization ensures that patients receive clear, comprehensible information about the clinical trial, boosting their engagement and understanding.
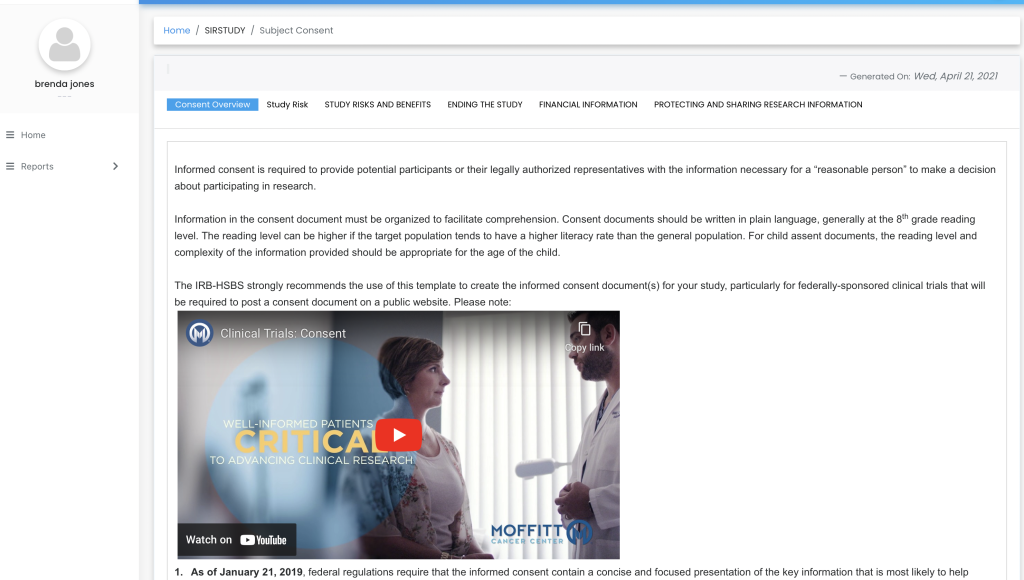
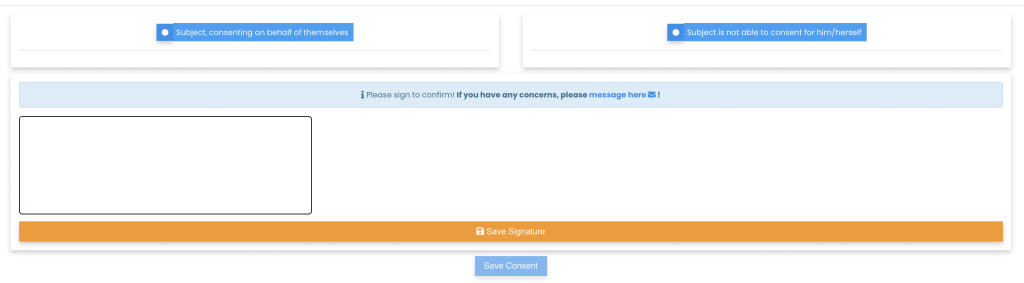
Remote Signatures
Patients and caregivers can sign off on the consent form remotely, using either hand signatures or video recordings. This flexibility supports patients who may have difficulty attending in-person meetings.
Monitoring
Our platform provides a single, user-friendly dashboard to monitor all patients’ consent, join video calls, and capture the status for each patient.
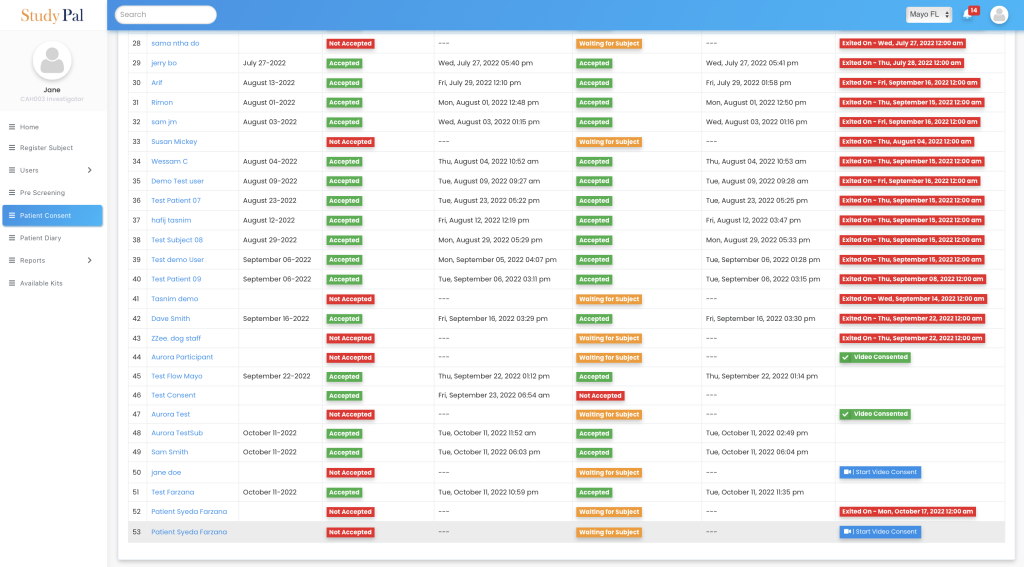

Comprehensive Support
Our eConsent software clinical trials allows caregivers to support patients during the consent process, ensuring that patients fully understand their commitments and responsibilities. This support is crucial for maintaining ethical standards and ensuring that patients make informed decisions.
DELVE HEALTH’S eCONSENT CLINICAL TRIAL PLATFORM IS UNLIKE ANY OTHER IN THE MARKETPLACE
Delve Health’s eConsent solution stands out in the marketplace due to its comprehensive features and patient-centric approach. Here’s what makes our platform unique:
Beyond Video Conferencing and DocuSign
Our eConsent goes beyond simple video conferencing or electronic signatures.
We provide a thorough, educational experience for patients, allowing them to ask questions and voice concerns with qualified study staff
Ethical and
Informed
Consent
We prioritize ethical standards by ensuring that patients are fully informed about their participation in the clinical trial. Our platform supports virtual conversations where patients can take the time to read and review documents at their own pace.
Compliance with
Privacy
Laws
Different countries have unique regulatory requirements, including privacy laws. Our eConsent tool helps your organization comply with these regulations, protecting patient data with advanced security features like facial recognition.
LET'S TALK
Integrating eConsent in Clinical Trials
Implementing eConsent in clinical trials can lead to a transformative experience for study participants. By providing simple, easy-to-comprehend clinical trial information, you can boost patient engagement and improve the overall patient experience.
Here’s how you can integrate Delve Health’s Clinical StudyPal platform into your trials:
Request a Demonstration
To learn more about our eConsent solution and how it can be tailored to your specific study needs, request a demonstration of the Clinical StudyPal platform. Our team will guide you through the features and benefits of our eConsent service, helping you design a hybrid or decentralized clinical trial that meets your goals.

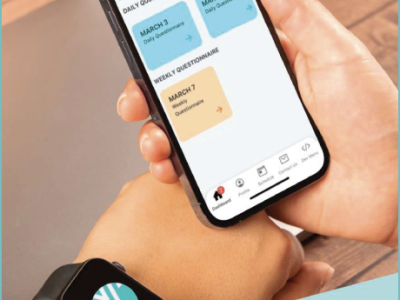
Seamless Integration
Our platform is designed for easy integration into your existing clinical trial processes. Whether you are conducting a traditional, hybrid, or fully decentralized trial, Delve Health’s eConsent solution can be seamlessly incorporated, providing a cohesive and efficient workflow.
Enhance Patient Engagement
By using our eConsent platform, you can provide a better patient experience, increasing engagement and compliance. The user-friendly interface, combined with interactive and multimedia elements, ensures that patients are well-informed and comfortable with their participation.


The Future of eConsent For Clinical Trials with Delve Health
Delve Health is committed to advancing the field of clinical trials through innovative technology and patient-centric solutions. Our eConsent platform is just one example of how we are transforming the clinical trial landscape. By automating and optimizing the consent process, we help organizations conduct more efficient, ethical, and compliant trials.
Why Choose Delve Health For Patient eCONSENT Clinical Trials?
Expertise in Clinical Trials
With years of experience in the field, Delve Health understands the unique challenges of clinical trials and provides tailored solutions to meet those needs.
Cutting-Edge Technology
Our platform leverages the latest technology to provide a seamless and efficient experience for both patients and researchers.
Patient-Centric Approach
We prioritize the patient experience, ensuring that participants are fully informed and comfortable with their involvement in the trial.
Compliance and Security
Our platform meets the highest standards of compliance and security, protecting patient data and ensuring regulatory adherence.
Frequently Asked Questions
eConsent in clinical trials is a digital process that allows patients to provide informed consent electronically. This method leverages technology to streamline and enhance the consent process, making it more efficient, secure, and user-friendly.
eConsent offers several benefits for clinical trials, including:
- Reduced costs through automation
- Faster study start-up processes
- Fewer informed consent errors
- Compliance with various regulatory requirements
- Multilingual capabilities for diverse patient populations
- Remote consent monitoring
- Improved patient compliance and retention
- Enhanced security features such as facial recognition
Yes, patients can sign the consent form remotely using either hand signatures or video consent. This flexibility allows patients to participate from any location, facilitating broader participation in clinical trials.
Delve Health ensures the security of patient data through advanced security features, including facial recognition technology. Our platform is designed to comply with local privacy laws and regulatory requirements, protecting sensitive patient information.
Delve Health’s eConsent platform stands out due to its comprehensive and patient-centric approach. Beyond simple video conferencing or electronic signatures, our platform offers thorough educational experiences for patients, virtual conversations with qualified study staff, and compliance with global privacy laws.
Integrating eConsent into your clinical trial is straightforward with Delve Health’s Clinical StudyPal platform. You can request a demonstration to learn more about the features and benefits of our eConsent service, and our team will guide you through the integration process.
Remote patient monitoring software is a tool that allows continuous monitoring of patient health data in real-time. It is essential for managing clinical trials, especially decentralized ones. When combined with eConsent, it ensures comprehensive patient management and adherence to trial protocols.
Yes, the eConsent process can be fully customized to meet the specific needs of different studies. You can add multimedia elements, such as videos and links to FAQs, to enhance patient understanding and engagement.
eConsent improves patient engagement by providing a user-friendly, interactive interface that makes the consent process easier to understand. Features like multimedia content and virtual conversations with study staff help patients feel more informed and involved in the trial.
Delve Health provides comprehensive support during the consent process, including virtual conversations with qualified study staff, customizable multimedia content, and real-time monitoring through our user-friendly dashboard.
The eConsent process can include a variety of multimedia elements such as videos, infographics, interactive content, and links to FAQs. These elements help enhance patient understanding and engagement, making the consent process more effective.




























