SERVICES
EPRO/ECOA CLINICAL TRIALS OUTCOME ASSESSMENT
We leverage cutting-edge technology to optimize data collection and patient engagement in clinical trials.
What are ePRO and eCOA in Clinical Studies?
ePRO and eCOA are digital methods for collecting patient-reported outcomes and clinical outcome assessments in clinical trials. These tools allow patients, clinicians, and caregivers to directly report outcomes using electronic devices, such as smartphones, tablets, and wearables. This hybrid model can include BYOD (Bring Your Own Device) or fully configured devices provided by the trial, enabling real-time data collection and more granular endpoint data.
Components of eCOA
- ePRO (electronic Patient Reported Outcomes): Patients report their own health status, symptoms, and overall well-being.
- ePerfO (electronic Performance Outcomes): Patients perform tasks or activities, and the performance data is captured electronically.
- eClinRO (electronic Clinician-Reported Outcomes): Clinicians record their observations and assessments electronically.
- eObsRO (electronic Observer-Reported Outcomes): Caregivers or observers report their observations about the patient’s condition.
LET'S TALK
The Evolution of Patient-Reported Outcomes
In recent years, there has been a significant shift towards patient-centric clinical trial designs. Approximately one-quarter of clinical trials today include patient-reported outcomes, a considerable increase from past decades. In 2014, about half of clinical trials still relied on paper forms for data collection. However, advancements in technology have demonstrated that electronic data collection not only improves the patient experience but also enhances data quality and reduces environmental impact by minimizing paper use.
Implementing ePRO and eCOA in Clinical Trials
The implementation of ePRO and eCOA in clinical trials begins with thoughtful trial design. Here’s how it works:
- Define Data Points and Feedback: The clinical research team identifies the specific data points and feedback required from patients.
- Choose the Best Format: The team decides on the best format for data collection, which may include an app, e-patient diary, wearable device, or other digital forms.
- Device Provision: Patients can either use devices provided by the trial or install an app like Clinical Study Pal on their own devices.
- Remote Monitoring: Once the ePRO/eCOA forms are completed, the research site can remotely monitor patient responses, enhancing patient compliance and overall trial experience.

Benefits of Using ePRO and eCOA in Clinical Trials

The advantages of incorporating ePRO and eCOA into clinical trials are manifold. These include:
Real-Time Data Capture
Certain measures, such as pain symptoms or daily activity levels, are better tracked in real-time rather than at scheduled site visits. Patient-reported outcomes provide the perfect solution for capturing this data accurately and promptly.
Higher Quality Data
Research indicates that electronic collection of PROs and COAs leads to higher quality data. For example, a study on a new treatment for overactive bladder demonstrated that using e-diaries for patients to track their urges to urinate resulted in a 33% reduction in data variability compared to paper diaries, significantly enhancing the study’s statistical significance.
Patient Preference
Patients generally prefer using ePRO apps over traditional paper diaries. A study by Almac in 2013 found that over 75% of patients favored answering questions electronically, underscoring the importance of adopting patient-friendly technology in clinical trials.
Enhanced Compliance and Engagement
A user-friendly digital platform enhances patient compliance and engagement. Patients can complete assessments conveniently on their own devices, ensuring they stay involved and committed to the trial.

Delve Health’s ePRO and eCOA Solutions
Delve Health offers state-of-the-art eCOA and ePRO solutions through our Clinical Study Pal platform. Here’s why our solutions stand out:

Customizable and Patient-Centric
Our eCOA and ePRO solutions are fully customizable to meet the specific needs of your clinical trial. You can incorporate multimedia elements, interactive features, and user-friendly interfaces to enhance patient understanding and engagement.
Comprehensive Monitoring
Our platform provides robust remote monitoring capabilities, allowing research sites to track patient responses in real-time. This feature ensures timely data collection and helps identify any compliance issues early on.


Environmentally Friendly
By reducing the reliance on paper forms, our eCOA and ePRO solutions contribute to environmental sustainability, making your clinical trials greener and more efficient.
High Security Standards
We prioritize the security of patient data with advanced encryption and compliance with regulatory standards. Our platform ensures that sensitive information is protected throughout the trial.


Multi-Device Compatibility
Our eCOA solutions are compatible with a wide range of devices, including smartphones, tablets, and wearables. This flexibility ensures that patients can use the devices they are most comfortable with, whether provided by the trial or their own.
Interactive and Intuitive Interface
The user interface of our eCOA solutions is designed to be intuitive and engaging, making it easy for patients to complete assessments and report outcomes. Interactive elements and clear instructions ensure that patients understand the process and provide accurate data.


Real-Time Data Access
eCOA research teams have real-time access to patient data, allowing for prompt analysis and decision-making. This real-time access also enables timely interventions if any issues arise, ensuring patient safety and trial integrity.
Comprehensive Support
Delve Health provides comprehensive support throughout the trial, from initial setup to ongoing monitoring and troubleshooting. Our team is dedicated to ensuring that your clinical trial runs smoothly and efficiently, leveraging our eCOA solutions to their fullest potential.
![ePRO / eCOA Delve Health Story [preview]](https://delvehealth.com/wp-content/uploads/elementor/thumbs/Delve-Health-Story-preview-qb3wg4nu7z3dg3c7skib8w00pxsc6kc828p0wtivgw.gif)
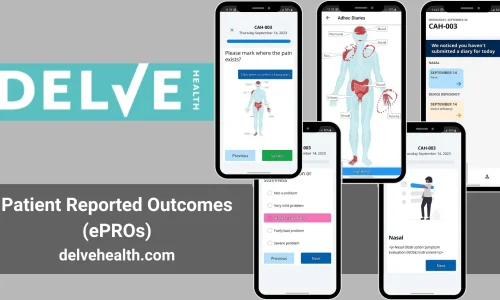
The Future of eCOA/ePRO for Clinical Trials
Delve Health is at the forefront of transforming clinical trials through the use of advanced electronic data capture methods. Our ePRO/eCOA platform is designed to provide accurate, real-time data while ensuring a patient-centric approach. By incorporating our solutions into your clinical trials, you can achieve higher data quality, better patient compliance, and a more efficient research process.
Why Choose Delve Health for ePRO and eCOA Clinical Trials?
Expertise in Clinical Research
With extensive experience in clinical research, Delve Health understands the unique challenges and requirements of conducting successful clinical trials. Our ePRO and eCOA solutions are designed to address these challenges and enhance the overall trial experience.
Commitment to
Innovation
We are committed to continuous innovation, constantly updating our platforms to incorporate the latest technological advancements and best practices in clinical research. This commitment ensures that our solutions remain at the forefront of the industry.
Patient-Centric
Approach
Our focus on patient centricity ensures that our solutions are designed with the patient in mind, making the trial experience as smooth and engaging as possible. We prioritize patient comfort and convenience, leading to higher compliance and better data quality
Frequently Asked Questions
ePRO stands for electronic Patient-Reported Outcomes, where patients use digital tools to report their health status, symptoms, and treatment experiences. This method provides real-time, accurate data directly from the patient, essential for evaluating the efficacy and safety of treatments.
Delve Health’s platform allows patients to report their outcomes using a digital device, either provided by the trial or their own. The data is captured in real-time and can be monitored remotely by clinical trial sites, ensuring accurate and timely data collection.
Yes, patients can use their own devices to report outcomes via the ePRO/eCOA platform. This BYOD (Bring Your Own Device) approach ensures flexibility and convenience for patients, making it easier for them to participate in the trial.
eCOA, or electronic Clinical Outcome Assessment, is a digital method for collecting outcome data directly from patients, clinicians, or caregivers in a clinical trial setting. eCOA encompasses various types of outcomes, including ePRO, ePerfO, eClinRO, and eObsRO.
By providing a user-friendly interface and real-time data collection, ePRO/eCOA makes it easier for patients to complete their assessments accurately and on time. This leads to higher compliance rates and better overall data quality.
It can capture a wide range of outcomes, including –
- Electronic Patient reported outcomes (ePRO)
- Electronic Performance outcomes (ePerfO)
- Electronic Clinician-reported outcomes (eClinRO)
- Electronic Observer-reported outcomes (eObsRO).
This comprehensive data collection ensures that all relevant clinical information is gathered.
Delve Health’s platform employs advanced security measures to protect patient data, including encryption and secure data storage. This ensures that all sensitive information is kept confidential and compliant with regulatory standards.
Integrating ePRO/eCOA into a clinical trial involves designing the trial to include –
- electronic data capture
- selecting the appropriate devices or apps
- training patients and clinicians on how to use the platform.
Delve Health provides comprehensive support throughout this process to ensure smooth implementation.
By replacing paper forms with electronic data capture, ePRO/eCOA significantly reduces the amount of paper used in clinical trials. This contributes to more sustainable research practices and minimizes the environmental footprint of the trial.




























