Background
A leading medical device company conducted a clinical trial to evaluate the efficacy of its new hypertension management device. The company faced challenges in ensuring patient adherence, particularly with wear compliance, managing follow-up with numerous patients, and collecting accurate blood pressure data from patients at home. The goal was to improve patient engagement, streamline data collection, and reduce operational costs while validating the effectiveness of their innovative device.
Challenges
- Wear Compliance: Ensuring patients consistently wore the hypertension management device and adhered to the clinical trial protocol.
- Large-Scale Patient Follow-Up: Managing the follow-up process with many patients, requiring substantial administrative resources.
- Remote Data Collection: Collecting accurate and timely blood pressure readings from patients at home, ensuring data reliability and validity.
Solution
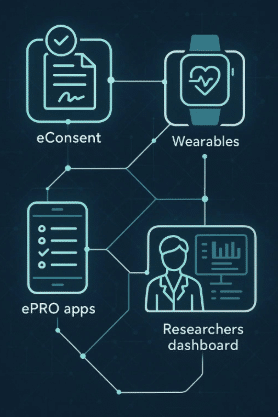
The medical device company partnered with Delve Health to leverage their advanced eCOA solutions and wearable technology. Delve Health provided a seamless platform to address the clinical trial’s challenges:
- Automated Patient Engagement: Delve Health’s platform automated all patient engagement activities, including reminders, notifications, and educational content, keeping patients informed and motivated. Patients received periodic messages reminding them when to wear the device and when PRO data needed to be collected.
- Remote Data Collection: Patients were equipped with wearable devices to monitor and report their blood pressure remotely. The device synced seamlessly with the Delve Health platform, ensuring real-time data transmission without requiring patients to remember when or how to perform tasks.
- Seamless PRO Collection: The platform facilitated the seamless collection of patient-reported outcomes (PROs), ensuring data accuracy and reliability. Patients could easily log their symptoms, medication adherence, and other relevant health information through a user-friendly app.
- White-Labeled Mobile App: Delve Health white-labeled a personalized mobile app for the company, enhancing patient understanding and trust by clearly identifying who the app and clinical trial were conducted for.
- Device Provision and Management: Delve Health provided the wearable devices and managed the entire distribution process. This ensured that each patient received their device promptly and was ready to participate in the clinical trial without delays.
Implementation Process
- Initial Setup: Delve Health conducted a comprehensive needs assessment to tailor their platform to the specific requirements of the hypertension clinical trial. This included configuring the wearable devices, setting up automated engagement protocols, and ensuring seamless integration with existing systems.
- Simplified Training and Support: Delve Health provided straightforward training sessions for the clinical site staff and deployed Delve concierge team members to provide high-touch patient support. The automated system made it easy for patients to conduct the clinical trial without extensive training. Continuous support was available to address any issues or questions.
- Deployment: Delve Health managed the distribution of wearable devices to patients and activated the automated engagement system. Regular follow-ups and data synchronization ensured that the clinical trial proceeded smoothly without significant interruptions.
Results
- High Patient Adherence: Patient adherence reached an impressive 98%, significantly higher than industry averages. The automated engagement system ensured patients remained compliant with clinical trial protocols and wore the device as required.
- Effective Remote Data Collection: Patients successfully collected and reported their blood pressure data remotely, eliminating the need for frequent site visits. This approach ensured timely and accurate data collection.
- Reduced Site Burden: The automation of patient engagement and PRO collection reduced the administrative burden on clinical sites by 80%. Staff could focus more on critical research activities rather than administrative tasks.
- Cost Savings: The clinical trial experienced a 28.8% reduction in costs due to streamlined processes, reduced need for site visits, and optimized data management.
Conclusion
The partnership with Delve Health proved transformative for the hypertension clinical trial. By utilizing Delve Health’s advanced eCOA solutions and wearable technology, the medical device company achieved outstanding results in patient adherence, data collection, site burden reduction, and cost savings.
Client Testimonial
“Partnering with Delve Health was a game-changer for our hypertension clinical trial. Their automated engagement system, remote data collection tools, and white-labeled mobile app ensured high patient adherence and significantly reduced our site burden. We are thrilled with the cost savings and the overall efficiency their platform brought to our trial.”