Introduction
In recent years, the emphasis on patient-centricity in clinical trials has grown substantially. With increasing demands for better patient engagement, satisfaction, and accessibility, technology plays a pivotal role in transforming how trials are conducted. Delve Health’s ePRO (electronic Patient Reported Outcomes) and eCOA (electronic Clinical Outcome Assessment) platforms are at the forefront of this evolution, providing comprehensive tools to foster patient-centric approaches. This blog explores how ePRO/eCOA technologies are reshaping clinical trial design to prioritize patient needs and improve outcomes.
What is eCOA in Clinical Trials?
Electronic Clinical Outcome Assessments (eCOAs) refer to digital tools that capture clinical trial data directly from patients, clinicians, and observers. These tools include electronic patient-reported outcomes (ePROs), clinician-reported outcomes (ClinROs), and observer-reported outcomes (ObsROs). The transition from paper-based to digital methods improves data accuracy, patient compliance, and overall trial efficiency.
The Need for Patient-Centric Trials
Evolving Regulatory and Market Demands
The shift toward patient-centered care in healthcare has echoed in the clinical research landscape. Regulatory bodies such as the FDA and EMA have emphasized the importance of involving patients more actively in clinical development processes. As a result, sponsors are focusing on trial designs that minimize patient burden, support engagement, and promote better retention.
Challenges in Traditional Clinical Trials
Traditional trials often require frequent site visits, paper forms, and long wait times, leading to decreased patient adherence and high dropout rates. Lack of real-time communication and feedback loops further alienate patients. These limitations necessitate a redesign of trial structures using technology that empowers and supports participants throughout their journey.
Leveraging ePRO/eCOA for Patient-Centric Trial Design
Enhanced Patient Experience
Delve Health’s ePRO/eCOA tools are designed with intuitive user interfaces, allowing patients to seamlessly input health data, track progress, and receive feedback. Mobile app compatibility, such as with Clinical StudyPal, ensures that patients can participate from the comfort of their homes, reducing travel-related stress.
Personalized Data Collection
Digital platforms offer the ability to tailor data collection schedules and formats to individual patient needs. Features such as reminder notifications, adjustable question sets, and multimedia content (e.g., videos or voice prompts) make it easier for participants to provide accurate and timely responses.
Remote Participation and Accessibility
Remote data entry enables patients from diverse geographical regions to join trials without relocating or commuting. Delve Health’s ePRO/eCOA systems also include multilingual support in 65+ languages and cultural localization, ensuring inclusion for patients of different backgrounds — a crucial factor in diverse and global studies.
Integration with Wearable Sensors and Remote Monitoring
Wearable Sensors in Clinical Trials
The integration of wearable sensors in clinical trials facilitates continuous health monitoring and data collection. Metrics such as heart rate, sleep patterns, and physical activity can be synced with ePRO/eCOA platforms, offering real-time insight into patient health.
Improved Data Accuracy and Timeliness
Combining subjective ePRO inputs with objective sensor data enhances data robustness. For instance, a patient reporting fatigue can be correlated with decreased physical activity captured by a wearable device. This dual-input model significantly improves clinical decision-making and data integrity.
Real-time Alerts and Adaptive Monitoring
The use of wearable technology allows for real-time alerts to both patients and trial coordinators. These alerts can flag abnormal readings or missed entries, allowing for timely interventions and better patient safety.
Delve Health Differentiator: Unlike many vendors that treat wearables as “just another ePRO field,” Delve Health built a dedicated wearable harmonization layer that converts commercial device signals into research-grade, regulator-ready endpoints. This allows sponsors to leverage devices patients already use (Apple, Samsung, Withings, etc.) without compromising compliance or data integrity.
Implementing Effective Patient Retention Strategies in Clinical Trials
Patient Concierge Support
Providing concierge services improves patient retention by offering individualized assistance throughout the trial. Services may include transportation coordination, appointment reminders, and real-time tech support—all integrated within the eCOA/ePRO platforms. Delve Health goes further by embedding remote navigators trained in health engagement who ensure patients remain connected throughout the study.
Feedback Loops and Patient Empowerment
Patients are more likely to remain engaged when they understand how their contributions affect the study. Delve Health enables participants to view study progress summaries and receive personalized feedback, fostering a sense of involvement and value.
Gamification and Motivation Tools
Incorporating elements of gamification such as rewards, progress badges, and challenge goals can improve engagement. These tools serve as motivational anchors, especially in long-term trials where sustained involvement is critical.
Mini Case Example: In a recent cardiovascular post-market study, Delve Health’s combination of ePRO/eCOA and wearables allowed patients to reduce site visits from monthly to just two over a 6-month follow-up — while maintaining retention rates above 85%.
Optimizing Trial Design for Patient Experience
Flexible Scheduling and Data Entry
Delve Health’s platforms allow patients to enter data at times that suit them best, removing the rigidity of in-person visits. Adaptive scheduling respects patient routines and increases adherence.
Multilingual and Cultural Sensitivity
For multinational trials, ePRO/eCOA systems support localization, ensuring content is linguistically and culturally appropriate. This improves comprehension and reduces errors due to miscommunication.
Simplified Onboarding with eConsent
Delve Health’s eConsent for clinical trials transforms the informed consent process into an interactive and user-friendly experience. Patients can review study materials through multimedia content, ask questions online, and electronically sign consent forms, streamlining enrollment and ensuring understanding.
Enhancing Data Integrity and Compliance
Improved GCP Compliance
Digital platforms inherently support compliance with Good Clinical Practice (GCP) guidelines. Audit trails, electronic signatures, and timestamped entries enhance accountability.
Error Reduction
Real-time data validation and logic checks prevent erroneous or incomplete entries. Built-in query resolution tools ensure that any discrepancies are addressed promptly.
Centralized Data Management
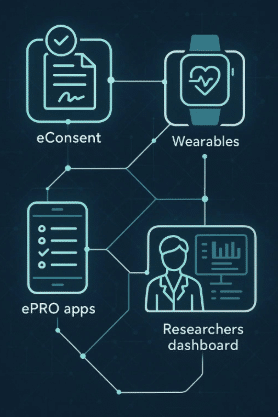
Delve Health’s unified platform enables consolidation of vendors by integrating ePRO/eCOA, wearables, concierge, and eConsent into one system. This reduces administrative burden, minimizes costs, and provides a single operational source of truth.
Conclusion
Delve Health’s ePRO/eCOA technologies represent a significant leap toward designing clinical trials that prioritize the patient experience. From simplifying data entry to supporting remote monitoring, personalized engagement, and robust compliance, these tools empower trial sponsors to conduct efficient, inclusive, and patient-focused studies.
By leveraging wearable harmonization, multilingual inclusivity, concierge support, and eConsent, Delve Health not only improves engagement and retention but also eliminates the complexity of working with multiple vendors. The result: faster studies, higher-quality data, and a truly patient-centric clinical trial model.
For more information about how Delve Health can help you implement patient-centric ePRO/eCOA solutions, [Contact us].
FAQs
What is eCOA in clinical trials?
eCOA stands for electronic Clinical Outcome Assessment. It refers to the use of electronic systems and devices to capture data directly from patients, clinicians, or observers during a clinical trial. This includes patient-reported outcomes (ePRO), clinician-reported outcomes (ClinRO), observer-reported outcomes (ObsRO), and performance outcomes (PerfO). eCOA helps improve data accuracy, supports regulatory compliance, and enhances the patient experience.
How does ePRO differ from traditional data collection methods?
ePRO (electronic Patient Reported Outcomes) allows patients to input their symptoms, treatment adherence, and quality of life data via smartphones, tablets, or other digital devices. This contrasts with traditional paper-based methods, which are prone to data entry errors and delays. ePRO enhances data timeliness, improves patient compliance, and facilitates remote monitoring in decentralized and hybrid trial settings.
Why is patient centricity important in clinical trials?
Patient centricity focuses on designing clinical trials that are accessible, inclusive, and respectful of patient needs and preferences. This approach enhances patient satisfaction, engagement, and retention in clinical trials. Patient-centric trials often result in better data quality and faster study completion due to higher participant adherence.
How can wearable sensors improve patient-centric trials?
Wearable sensors in clinical trials allow for real-time data collection on vital signs, activity levels, sleep patterns, and other health metrics. These devices reduce the need for site visits and support remote participation, making trials more convenient and less intrusive for patients. Wearable sensors also provide continuous, objective data that enhances trial outcomes.
How does eConsent support patient-centricity?
eConsent for clinical trials enables participants to review and sign consent forms electronically. This process often includes multimedia content such as videos or interactive Q&A to enhance understanding. eConsent promotes transparency, improves comprehension, and allows patients to make informed decisions from the comfort of their home.