Designing Patient-Centric Trials with ePRO/eCOA Technologies
Introduction
Patient-centricity in clinical trials has shifted from buzzword to business necessity. Regulators, sponsors, and — most importantly — patients are demanding trials that are more inclusive, accessible, and less burdensome.
Yet while many vendors talk about “patient centricity,” most simply digitize old processes. At Delve Health, we believe true patient-centricity comes from rethinking the entire ecosystem — from how data is captured, to how patients are engaged, to how sponsors consolidate vendors.
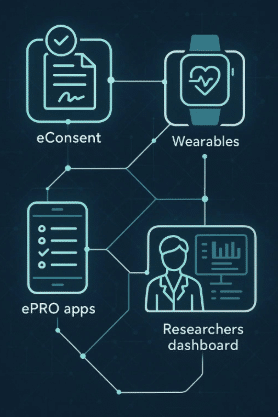
Our ePRO (electronic Patient Reported Outcomes) and eCOA (electronic Clinical Outcome Assessment) platforms are more than digital forms. They are the connective tissue between patients, sites, and sponsors. This blog explores how Delve Health’s unique approach is reshaping clinical trial design.
What is eCOA in Clinical Trials?
eCOA encompasses digital tools that capture outcomes directly from patients, clinicians, and observers — including ePRO, ClinRO, ObsRO, and PerfO.
Every vendor can give you a digital survey. The difference lies in what you do with the data. At Delve Health, eCOA isn’t just about collection — it’s about integration with wearables, concierge support, and real-time engagement so data becomes usable evidence, not just another dataset.
Why Patient-Centric Trials Still Fall Short
The Industry Problem
Most clinical trials still place a heavy burden on patients — multiple site visits, repetitive questionnaires, poor feedback loops. Even when digitized, these processes often mirror paper workflows instead of reimagining them.
Regulatory Push
The FDA and EMA have been clear: patients must be at the center of trial design. Yet many platforms lag behind, treating digital tools as bolt-ons. That’s why adoption is slow, and dropout rates remain stubbornly high.
Delve’s Approach: Beyond Digital Forms
Enhanced Patient Experience
Delve Health’s ePRO/eCOA platforms (including Clinical StudyPal) are designed for usability. Patients can report outcomes on mobile devices, view progress, and receive tailored feedback — all without the stress of constant site visits.
Case Example: In a cardiovascular post-market study, shifting from paper diaries to Delve’s mobile ePRO tools cut site visits from monthly to just two in six months — while maintaining retention above 85%.
Personalized, Inclusive Data Capture
Every patient is different. Our platform allows adaptive scheduling, multimedia prompts, and multilingual support in 65+ languages, making trials inclusive for global and underserved populations.
Wearable Harmonization: A True Differentiator
Here’s where most vendors get it wrong: they treat wearables like “just another ePRO field.” But wearables generate continuous, dynamic signals that need validation and harmonization.
Delve Health built a wearable harmonization layer that transforms commercial device data (Apple, Samsung, Withings, etc.) into research-grade, regulator-ready endpoints. Sponsors don’t have to choose between consumer usability and compliance — they get both.
Concierge Support for Retention
Patient centricity isn’t just digital. Delve integrates concierge services and remote navigators directly into our platform. Whether it’s helping a patient troubleshoot an app, coordinating transportation, or reminding them of an assessment, our navigators are the human layer that drives retention.
Case Example: In a neurology study, our bilingual English–Spanish concierge team kept rural patients engaged who otherwise would have dropped out due to travel and language barriers.
Gamification and Feedback Loops
Patients stay engaged when they feel valued. Delve’s platform gives participants progress summaries, motivational nudges, and optional gamification features (badges, rewards, challenges). Engagement isn’t left to chance — it’s designed in.
Trial Design Optimized for Patients and Sponsors
- Flexible Scheduling → Patients enter data on their own time.
- Cultural Sensitivity → True localization beyond translation.
- Simplified Onboarding → Interactive eConsent that educates, not intimidates.
- Vendor Consolidation → One platform for ePRO, eCOA, wearables, concierge, and eConsent. Sponsors spend less time managing contracts and integrations — and more time on science.
Data Integrity and Compliance Built In
- Regulator-Ready → Fully compliant with FDA, EMA, HIPAA, GDPR, and 21 CFR Part 11.
- Audit Trails → Timestamped entries and electronic signatures.
- Error Reduction → Real-time validation checks, logic rules, and centralized data monitoring.
Conclusion
Patient-centricity is not about digitizing paper. It’s about removing barriers, reducing burden, and respecting patient lives while giving sponsors higher-quality data.
Delve Health’s ePRO/eCOA solutions go further by unifying digital tools, wearables, concierge, and multilingual engagement into one research-ready ecosystem. That’s how trials become truly patient-centric — and why sponsors looking to reduce dropout, improve compliance, and accelerate approvals are choosing Delve.
👉 Ready to make your next trial truly patient-first? [Contact us].
FAQs
What is eCOA in clinical trials?
eCOA (electronic Clinical Outcome Assessment) refers to the electronic capture of patient-reported, clinician-reported, or observer-reported outcomes using digital tools. It improves data accuracy, patient engagement, and regulatory compliance.
How do wearable sensors benefit clinical trials?
Wearable sensors in clinical trials allow continuous, passive monitoring of patient health data, enabling real-time insights and reducing the burden of frequent site visits.
What are effective patient retention strategies in clinical trials?
Effective strategies include offering concierge support, using engaging digital platforms, providing personalized feedback, and incorporating gamification and educational resources.
Why consolidate vendors?
Managing multiple point solutions increases cost and complexity. Delve integrates ePRO, eCOA, wearables, concierge, and eConsent into one ecosystem. For more information about how Delve Health can help you implement patient-centric ePRO/eCOA solutions, [Contact us].
What improves patient retention in trials?
Concierge support, multilingual engagement, personalized feedback, and gamification — all built into Delve’s platform.