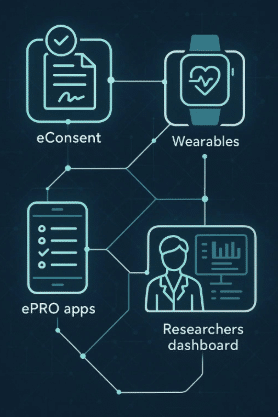
The use of technology has been increasing over the past few years, with devices such as wearables, biosensors, and other medical devices. One area where this has been particularly evident is the use of digital endpoints in clinical trials. Digital endpoints refer to data collected from digital sources, such as mobile apps and passively collected data via wearable devices; which are used to assess the efficacy of a treatment or intervention in a clinical trial. They allow more frequent, objective remote monitoring of patients during clinical studies. For example, a smartphone’s microphone might be used to diagnose or predict mild cognitive impairment due to Alzheimer’s disease or a wrist-worn activity monitor (such as those found in smartwatches) may be used to measure a drug’s effect on the nocturnal activity of patients with sickle cell disease (Source: PubMed).
It is important to note that there is a difference between outcomes and endpoints. For example, an endpoint is the primary outcome that is being measured by a clinical trial. A cancer drug, for example, might use survival as an endpoint, comparing the five-year survival rate of patients using an experimental therapy against the five-year survival rate of patients using another treatment or a placebo (Source: Friends of Cancer Research).
There are several benefits of using digital endpoints in clinical trials:
- Objective Data Collection: Digital endpoints provide objective and accurate data collection, as they are less prone to bias than traditional subjective methods of gathering data. For example, using wearable devices to measure a patient’s heart rate provides more accurate and reliable data than asking patients to self-report their heart rate.
- Increased Patient Engagement: The use of digital endpoints can increase patient engagement and adherence to treatment, as patients can monitor their own progress and receive real-time feedback on their health status. This engagement also leads to improved patient retention rates in clinical studies.
- Time and Cost Savings: Digital endpoints can save time and reduce costs in clinical trials as they eliminate the need for manual data entry, paper formats with associated storage costs or losses (i.e., automated workflows) and reduce the need for site visits by patients (i.e., remote patient monitoring, telehealth visits, etc.).
- Improved Data Quality: The use of digital endpoints can improve the quality of data collected in clinical studies, as digital data is more standardized and can be more easily validated and audited.
Challenges of Digital Endpoints in Clinical Trials
Despite the benefits of digital endpoints, there are also several challenges to their use in clinical trials that we need to acknowledge, along with our solutions:
- Data Privacy and Security: The use of digital endpoints raises concerns about data privacy and security, as sensitive health data is collected and stored electronically. Delve Health’s technology solution has developed security measures to protect patient data from unauthorized access or breaches that are also HIPPA, GDPR, and 21CFRpart11 compliant.
- Standardization of Data: There is a need for standardization of digital endpoint data across multiple devices and platforms to ensure consistency and comparability of data across trials. Delve Health’s technology solution is device agnostic and integrates with virtually any EDC platform and OS, in addition to being compliant with regulatory agencies and fully-customizable for each individual client and their specific clinical trial. Delve Health works together as an extension of your in-house team, partnered together to meet the same goals, ensures successful results.
- Data Validation: The validity and reliability of digital endpoint data needs to be established to safeguard the accurate health status of patients. This requires validation studies and comparison with traditional clinical endpoints. Delve Health can help automate the analysis of the data, safely and securely, working in tandem with your clinical study team.
- Regulatory Acceptance: Regulatory acceptance of digital endpoints in clinical studies is still evolving, and there is a need for guidance and standards from regulatory agencies to ensure their appropriate use. Again, Delve Health’s technology platform is HIPPA, GDPR and 21CFRpar11 compliant, so while we understand that things may change, our solution is agile and can quickly update and adapt to any new regulations that are introduced to our industry.
Conceptually, an ideal digital endpoint should be a valid and applicable measure of how a patient feels, functions and/or survives; as well as be perceived by end-users of the research as having meaning and value.
In clinical trials, an event or outcome that can be measured objectively to determine whether the intervention being studied is beneficial. Navigating the shift from traditional trial models to agile, patient-centric processes driven by digital health technologies, the use of digital endpoints in clinical trials has the potential to revolutionize the way clinical trials are conducted by providing objective data collection; increased patient engagement; time and cost savings; and improved data quality. However, their use also raises challenges related to data privacy and security, standardization of data, data validation, and regulatory acceptance—which is why integrating a platform that is HIPPA, GDPR and 21CFRpart11 compliant is extremely important.
Delve Health is in a unique position to help your clinical study team design a decentralized clinical trial; integrate wearable devices; implement a digital platform; passively gather and analyze high quality patient data; and execute a successful clinical trial, complete with increased patient engagement and satisfaction overall.