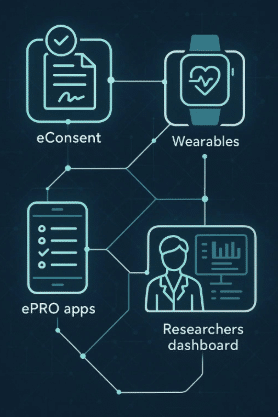
Digital measures in clinical trials are changing the game, offering tools like gait tracking and real-time data collection that accelerate drug development, reduce costs, and improve patient engagement.
Let’s be real—clinical trials are stuck in a time warp. In a world where digital health tools can track our steps, heartbeats, and even sleep patterns in real time, it’s mind-blowing that so many clinical trials still rely on outdated, paper-based processes and intermittent data. This old-school approach is slow, expensive, and doesn’t do justice to the incredible insights we could gain from continuous, real-world patient data. The good news? Digital measures are changing all of that.
At Delve Health, we’re on a mission to transform the clinical trial process using digital measures—tools like gait trackers, wearables, mobile apps, and other digital health technologies (DHTs). This isn’t just about upgrading tech; it’s about saving lives by getting life-saving drugs to patients faster. So, let’s break down how digital measures, particularly innovative tools like gait measurement, are advancing clinical trials, reducing costs, and moving us toward a new era in healthcare.
Why Digital Measures in Clinical Trials Matter: Real-Time Data for Real-Life Insights
In traditional clinical trials, data collection often depends on periodic visits to research sites. This gives researchers snapshots of a patient’s health, but it doesn’t capture the full picture. With digital measures, data is collected continuously, offering a 24/7 window into how a patient is responding to treatment. Imagine tracking mobility, sleep, heart rate, or glucose levels as they happen, without the need for constant clinic visits.
Take gait measurement, for example. Gait, or the way a person walks, is a key indicator for many diseases, including Parkinson’s, arthritis, and multiple sclerosis. Traditionally, measuring gait would require patients to come into a clinic, where a trained professional would assess their movements. Now, with wearable gait trackers, patients can go about their daily lives while the device collects data in real time. Researchers get a complete picture of how a treatment affects mobility, not just on a single day but every day.
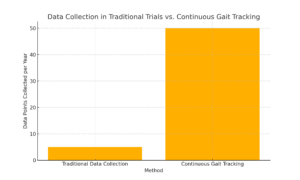
Gait Tracking and Digital Biomarkers in Clinical Trials: New Ways to Measure What Matters
Gait tracking is just the beginning. Digital measures allow us to capture a range of biomarkers that go far beyond what was possible before. We can now monitor things like heart rate variability, glucose levels, sleep patterns, and physical activity—all critical indicators of a patient’s health. This data gives researchers a high-resolution view of a patient’s response to treatment.
Imagine tracking heart rate patterns for a cardiology drug trial or using continuous glucose monitoring to refine diabetes treatments. The insights we gain from these digital biomarkers aren’t just cool tech—they’re powerful tools that can speed up trials, cut costs, and make clinical research more patient-centric.
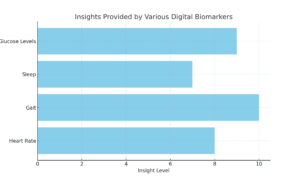
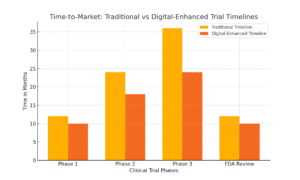
Faster Insights, Faster Adaptation—Cutting Trial Timelines
Speed is crucial in drug development. Every day saved in the clinical trial process means patients can access life-saving drugs sooner. With digital measures, researchers get immediate, real-world insights into a patient’s response, enabling adaptive trial designs. This means that instead of waiting weeks or months to gather enough data, researchers can analyze results in real time and adjust protocols as they go.
Let’s put it in perspective. Traditional trials often take years, partly because data collection is slow and decisions take time. With tools like gait tracking, researchers can spot treatment impacts immediately and even predict outcomes earlier. By making informed, real-time adjustments, they can cut down trial timelines, saving time and, ultimately, lives.
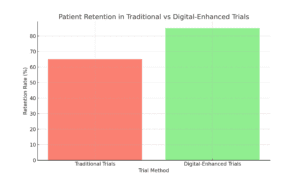
Engaging Patients, Improving Retention—Making Trials Easier and More Accessible
One of the biggest challenges in clinical trials is keeping patients engaged. Between lengthy clinic visits, complex protocols, and demanding schedules, patients often drop out, which disrupts data collection and slows down the entire trial process. Digital tools make it easier and more accessible for patients to stay connected without leaving home.
For example, a patient in a trial can wear a device that tracks gait or heart rate and receive automated reminders on their phone. This approach isn’t just convenient; it keeps patients engaged and motivated. When patients are more engaged, they’re more likely to stick around, and trials get the consistent, reliable data needed for success.
Cutting Costs and Increasing Efficiency—Maximizing Value with Every Dollar
Clinical trials are notoriously expensive, with costs easily reaching into the millions. Digital measures streamline operations and reduce costs by eliminating frequent site visits, automating data collection, and simplifying data analysis. For instance, wearable gait measurement devices allow continuous tracking without needing specialists to monitor gait in a clinical setting—saving time and cutting expenses.
Imagine a trial using remote devices that upload data automatically to a secure database. With automation in place, you’re cutting out the manual data entry and costly in-person visits. This makes trials more affordable, scalable, and faster to run, giving researchers the flexibility to focus resources where they matter most.
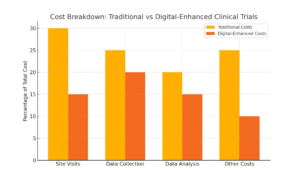
Improved Accuracy with Continuous, Real-World Data
Traditional clinical trials often rely on scheduled snapshots—data collected at a few time points that may miss critical fluctuations or responses. Digital measures offer continuous, real-world data, providing a more accurate view of how treatments impact patients in their everyday lives. With tools like gait trackers and heart rate monitors, we’re no longer limited to periodic measurements taken in artificial clinical settings. Instead, we get a full, nuanced picture of treatment effects, enhancing both accuracy and reliability.
For instance, in a Parkinson’s trial, gait tracking can reveal subtle improvements in a patient’s stability over time, something a weekly check-up might miss. This level of accuracy leads to stronger, more reliable conclusions, ultimately bringing better drugs to market faster.
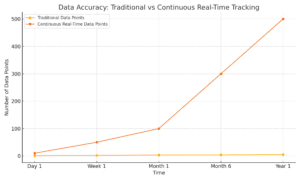
Accelerating Time-to-Market with Digital Measures in Clinical Trials
Time-to-market is more than a financial metric; it’s a matter of life and death. Every day we save in the drug development process is another day a patient gets access to a potential life-saving treatment. By using digital measures like gait tracking, we’re creating a future where life-saving treatments don’t get held up by outdated processes.
Digital measures make it possible to bring drugs to market faster by enabling adaptive trials, reducing costs, improving patient retention, and capturing more accurate data. At Delve Health, we’re using these tools to redefine clinical trials, helping researchers bring more effective drugs to patients sooner. The future of clinical trials isn’t just coming—it’s already here.
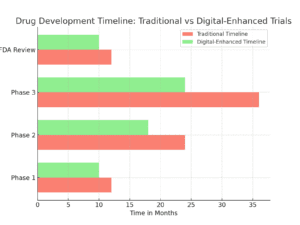
Conclusion: Digital Measures Are Transforming Clinical Trials—And It’s Just the Beginning
The future of drug development is bright with the integration of digital measures in clinical trials. Digital measures are transforming clinical research, from continuous gait tracking to heart rate monitoring and glucose tracking. They’re helping us bring life-saving drugs to market faster, cheaper, and with better data. This isn’t just about tech for tech’s sake—it’s about giving patients access to the treatments they need, sooner and more effectively.
At Delve Health, we’re at the forefront of this digital transformation. We believe that by embracing digital health tools, we’re not only making trials faster and more efficient; we’re improving lives. Join us on this journey as we empower clinical trials with digital measures, and let’s get life-saving drugs to patients without delay. The future is here, and it’s digital.
References:
To support the claims in this blog post, here are several credible references:
FDA Guidance on Digital Health Technologies: The U.S. Food and Drug Administration (FDA) has issued guidance on the use of digital health technologies for remote data acquisition in clinical investigations. This document provides recommendations for sponsors, investigators, and other stakeholders on the use of digital health technologies (DHTs) to acquire data remotely from participants in clinical investigations evaluating medical products. FDA
Study on Wearable Devices in Clinical Trials: A study published in BMC Medicine discusses the use of wearable biometric monitoring devices to measure outcomes in clinical trials. The research highlights how these devices offer the opportunity to collect biological, physiological, or behavioral patient data continuously, remotely, and unobtrusively, representing a revolution in clinical research and trials. BMC Medicine
Systematic Review on Wearable Devices in Oncology: A systematic review published in The Oncologistexamines the use of wearable devices in oncology patients. The review indicates that data provided from wearable devices could offer additional information to the medical team about a patient’s health state, facilitating better care. Oxford Academic
FDA Final Guidance on Digital Health Technologies: The FDA released final guidance on the use of digital health technologies for remote data acquisition in clinical investigations. This guidance provides recommendations on the use of DHTs to acquire data remotely from participants in clinical investigations that evaluate medical products. FDA
Article on Wearable Biosensors for Decentralized Clinical Trials: An article by Medpace discusses the use of wearable biosensors for decentralized and hybrid clinical trials. It highlights how the COVID-19 pandemic has accelerated the adoption of digital evolution in clinical research, emphasizing the current ecosystem of remote biosensor technology in clinical trials. Medpace
FDA Law Blog on Digital Health Technologies Guidance: An article from the FDA Law Blog discusses the FDA’s final guidance on the use of digital health technologies for remote data acquisition in clinical investigations. It provides insights into the FDA’s recommendations and the broader framework for digital health technologies. The FDA Law Blog
Yale HRPP Agency Guidance Snapshot: Yale University’s Human Research Protection Program provides a snapshot of the FDA’s final guidance on digital health technologies for remote data acquisition in clinical investigations. This document offers an overview of the guidance and its implications for clinical research. Yale University
FDA Draft Guidance on Digital Health Technologies: The FDA’s draft guidance on digital health technologies for remote data acquisition in clinical investigations provides recommendations for sponsors, investigators, and other stakeholders on the use of DHTs to acquire data remotely from participants in clinical investigations evaluating medical products. FDA
PLOS Digital Health Article on Ethical Landscape of Digital Biomarkers: An article published in PLOS Digital Health maps the ethical landscape of digital biomarkers, discussing their potential to revolutionize health monitoring, diagnosis, biomedical discovery, and drug development. PLOS Journals
Alzheimer’s & Dementia Journal Article on Digital Technologies as Biomarkers: An article in Alzheimer’s & Dementia: Translational Research & Clinical Interventions discusses how digital technologies are transforming clinical trials in various disease areas and emerging efforts to apply lessons learned to the Alzheimer’s disease clinical trial space. Alzheimer’s & Dementia