Per the FDA guidance, an electronic consent is any “the use of electronic systems and processes that may employ multiple electronic media to obtain informed consent for both HHS-regulated human subject research and FDA-regulated clinical investigations of medical products, including human drug and biological products, medical devices, and combinations thereof.”
eConsent is gaining greater acceptance and adoption in clinical trials
- 90% of top 10 pharma have an eConsent initiative in place
- 88% of top 25 pharma implemented eConsent
- 66% of top 50 pharma companies are engaged in planning an eConsent initiative
Every clinical trial is different, but implementing the right eConsent solution will improve your clinical trial startup through improved patient understanding, site satisfaction, and regulatory compliance.
96% of clinical trial participants that have used eConsent, prefer it over paper informed consent.
- Gaining popularity and higher satisfaction with elderly participants
- Combining multi-media with quiz aids better information
- More accurate, improves comprehension and makes better clinical trial patients
77% of sites mentioned that eConsent has improved patient consent and site startup.
- Improves time spent with patients
- Quickly address questions and responses to patients
- Better compliance and controlled document management
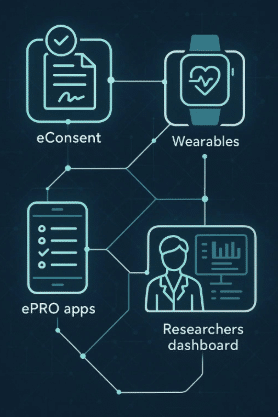
Delve Health’s Clinical StudyPal is a decentralized platform that can help engage sponsors, investigative sites, and patients. Collect the most pertinent information. We help improve patient compliance in clinical trials, create better consent modules, and integrate with EDC/CTMS/eTMF/Wearables for better clinical trial conduct.
Big pharma is already embracing eConsent. You can get the same benefits by talking with our experts at Delve Health. Reach out and talk to our experts on how to implement a cost-effective eConsent solution for your upcoming clinical trial.
Click here to schedule a call or call one of our experts at the numbers below
Delve Health https://delvehealth.com