Introduction
Diversity in clinical trials is essential for ensuring that medical research is applicable to all patient populations. Recently, the U.S. Food and Drug Administration (FDA) issued new guidance on Diversity Action Plans aimed at improving the enrollment of participants from underrepresented populations in clinical studies. Delve Health is dedicated to supporting these initiatives by promoting diversity and providing opportunities for underprivileged populations to participate in clinical research.
Understanding the FDA’s New Guidance
The FDA’s draft guidance, titled “Diversity Action Plans to Improve Enrollment of Participants from Underrepresented Populations in Clinical Studies,” assists medical product sponsors in creating and submitting Diversity Action Plans. These plans are crucial for increasing participation from historically underrepresented populations, thus enhancing the quality and relevance of clinical trial data.
Rationale Behind the FDA’s Guidance
Enhancing diversity in clinical trials ensures broader applicability of study results, providing valuable insights into the safe and effective use of medical products across diverse demographic groups. This approach helps in understanding the variations in disease manifestation and treatment response among different populations.
Details of the Diversity Action Plans
Diversity Action Plans must outline the sponsor’s rationale and goals for clinical study enrollment, categorized by age, ethnicity, sex, and race. The plans should describe strategies to achieve these goals, considering broader dimensions of diversity beyond basic demographics.
Regulatory Context and Requirements
The requirement for submitting Diversity Action Plans is part of new provisions in the Federal Food, Drug, and Cosmetic Act (FDORA). These plans apply to phase 3 clinical studies and other pivotal studies of drugs, biological products, and certain medical devices. Sponsors must submit these plans for studies whose enrollment begins 180 days after the final guidance publication.
Evaluation and Waiver Process
The FDA will evaluate Diversity Action Plans based on specified criteria and may grant waivers under certain conditions. Sponsors must provide a rationale for not submitting a Diversity Action Plan and demonstrate why it is not feasible or necessary for their specific study.
FDA’s Collaborative Efforts
The guidance was developed collaboratively by the FDA’s Oncology Center of Excellence, the Center for Drug Evaluation and Research, the Center for Biologics Evaluation and Research, the Center for Devices and Radiological Health, the Office of Women’s Health, and the Office of Minority Health and Health Equity. This multi-center approach ensures comprehensive and inclusive guidance.
Impact on Clinical Research
Diverse clinical trial populations enhance the applicability of research findings and improve public health outcomes. Case studies have shown that inclusive enrollment leads to better understanding of treatment effects across different demographic groups, ultimately resulting in more effective and safer medical products.
Delve Health’s Commitment to Diversity
Delve Health is dedicated to promoting diversity in clinical trials. By implementing strategies that encourage participation from underrepresented groups, Delve Health ensures that clinical trials are inclusive and representative. This commitment aligns with the FDA’s guidance and the broader goal of improving public health.
Supporting Underprivileged Populations
Delve Health actively works to include socio-economically disadvantaged groups in clinical trials. Through targeted programs, they provide access to clinical studies for underprivileged populations, ensuring that these groups benefit from the latest medical advancements.
Innovative Approaches by Delve Health
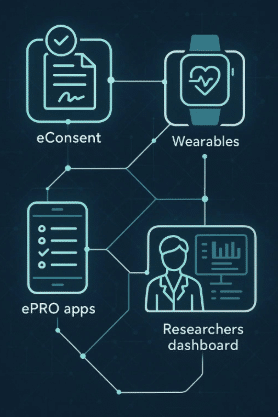
Delve Health utilizes cutting-edge technology to reach diverse populations. Their mobile health initiatives and remote monitoring solutions make it easier for participants from various backgrounds to engage in clinical studies. Telehealth services further enhance accessibility and convenience for all participants.
Future Directions and Goals
Looking ahead, Delve Health plans to further enhance its diversity initiatives. By setting long-term goals and commitments, Delve Health aims to continually improve the inclusivity of clinical research and contribute to better health outcomes for all populations.
Conclusion
The FDA’s new guidance on Diversity Action Plans marks a significant step towards more inclusive clinical research. Delve Health’s proactive efforts in promoting diversity and supporting underprivileged populations demonstrate their commitment to improving public health. By working together, we can ensure that clinical trials are representative and beneficial for everyone.
FAQs
What are Diversity Action Plans and why are they important?
- Diversity Action Plans are strategies developed by clinical study sponsors to ensure the inclusion of underrepresented populations in clinical trials. They are important for enhancing the relevance and safety of medical products for diverse patient groups.
How does the FDA’s guidance affect clinical trial sponsors?
- The FDA’s guidance requires sponsors to submit Diversity Action Plans for certain clinical studies, outlining their goals and strategies for inclusive enrollment. This helps ensure that clinical trial results are applicable to a broader range of patients.
What initiatives does Delve Health have in place to support diversity?
- Delve Health employs various strategies, including community outreach, use of technology, and partnerships with local organizations, to promote diversity in clinical studies and support underprivileged populations.