Parental Involvement in Pediatric Clinical Trials: Supporting Data Collection and Patient Care
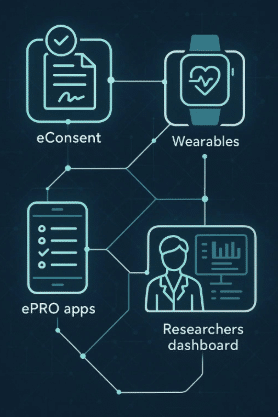
Pediatric clinical trials rely heavily on parental involvement to ensure the trial runs smoothly, from data collection to supporting patient care. Parents are not only responsible for providing informed consent but also play an active role in managing their child’s participation throughout the study. With technology like Delve Health’s Clinical StudyPal, parents can now more easily track symptoms, manage wearable devices, and communicate with research teams. This article will delve into how pediatric clinical trials benefit from parental involvement and the tools that make the process easier.
Why Parental Involvement is Crucial in Pediatric Clinical Trials
Children enrolled in pediatric clinical trials depend on their parents or guardians to facilitate participation. Here’s why their involvement is so essential:
- Informed Consent: Parents provide informed consent on behalf of their children, understanding the risks, benefits, and the commitment required throughout the study. Learn more about the importance of consent in pediatric trials from the FDA’s pediatric research guidelines (DoFollow external link).
- Data Reporting and Symptom Tracking: Parents are responsible for logging their child’s symptoms, tracking medication adherence, and reporting side effects. Their observations are crucial for accurate data collection in pediatric trials.
- Managing Wearables and Technology: Many modern trials use wearables to gather real-time health data. Parents must ensure these devices, such as the Apple Watch or Fitbit, are used properly for continuous data reporting. Find more details on wearables used in trials at ClinicalTrials.gov (DoFollow external link).
- Emotional Support: Participation in a trial can be emotionally taxing for children. Parents provide the psychological and emotional support needed to help their child adhere to the protocol while maintaining a sense of normalcy.
Challenges Parents Face During Pediatric Clinical Trials
Parents face several challenges while managing their child’s participation in pediatric clinical trials, such as:
- Balancing Responsibilities: Many parents juggle work, family obligations, and trial responsibilities. Frequent hospital visits and at-home monitoring add to their daily duties.
- Understanding Medical Information: The complexities of clinical trial protocols and medical information can overwhelm parents, especially when it involves interpreting medical data related to their child’s condition.
- Emotional Strain: Parents often carry the emotional weight of uncertainty about their child’s health, the success of the trial, or the potential side effects of the treatment.
- Managing Wearables and Data Reporting: Wearable devices like the Apple Watch and other health monitors provide real-time data but need proper management. Parents are responsible for ensuring the devices are used correctly and data is transmitted to the research team.
How Delve Health’s Clinical StudyPal Supports Parents in Pediatric Clinical Trials
Delve Health’s Clinical StudyPal offers a variety of solutions to ease the challenges parents face during pediatric clinical trials. By simplifying data collection and communication, Clinical StudyPal makes it easier for parents to manage their child’s participation in the trial without being overwhelmed.
Key Features of Clinical StudyPal:
- User-Friendly Mobile App: The app allows parents to log symptoms, track medication adherence, and manage wearable devices all in one place. This real-time data collection helps ensure that researchers have access to accurate, up-to-date information.
- Remote Monitoring & Data Collection: Clinical StudyPal integrates with wearables like the Apple Watch, Fitbit, and Oura Ring to monitor key health metrics such as heart rate and sleep patterns. This real-time data is automatically captured and transmitted, reducing the need for in-person visits.
- Streamlined Communication: Parents can communicate directly with research teams via Clinical StudyPal, allowing them to ask questions or report concerns about their child’s participation in the study quickly and efficiently. This simplifies communication, saving time and reducing the stress of managing a trial.
- Simplified Consent Process: If a child transitions from pediatric to adult care during a trial, Clinical StudyPal simplifies the re-consent process, ensuring parents can easily manage this transition with digital tools.
- Educational Resources: Clinical StudyPal provides access to educational materials to help parents understand the trial protocols and best support their child during the trial process.
The Importance of Communication Between Parents and Researchers
Clear and open communication between parents and researchers is key to ensuring pediatric clinical trials run smoothly. Parents need to feel empowered to share feedback, report symptoms, and ask questions. With Clinical StudyPal, this communication is streamlined, allowing for quicker and more efficient updates between both parties.
Tips for Effective Communication:
- Stay Informed: Parents should review the trial’s protocols regularly and stay informed about any updates or changes.
- Ask Questions: When in doubt, parents should reach out to the trial team for clarification.
- Report Changes Promptly: Any significant changes in a child’s symptoms or reactions to treatment should be reported as soon as possible to ensure the safety of the participant and the integrity of the data.
Leveraging Wearables in Pediatric Clinical Trials
Wearable technology like the Apple Watch, Fitbit, and Oura Ring allows for seamless, non-invasive data collection in pediatric trials. These devices are crucial for monitoring health metrics without the need for frequent in-person visits, making them a valuable tool for both researchers and parents.
Parents are responsible for ensuring that these devices are properly worn and the data is transmitted correctly. Clinical StudyPal simplifies this by automating much of the data collection, ensuring accuracy and compliance.
Conclusion
Parental involvement is vital to the success of pediatric clinical trials. While parents face several challenges, platforms like Delve Health’s Clinical StudyPal are helping ease their burden by offering intuitive tools for data collection, communication, and managing their child’s participation. With technology and real-time data from wearable devices like the Apple Watch, parents can feel more confident in supporting their child through the clinical trial process.
FAQs
- Why are parents essential in pediatric clinical trials?
Parents provide consent, track symptoms, and ensure their child follows the study protocols, playing a crucial role in data collection. - How does Clinical StudyPal make trials easier for parents?
Clinical StudyPal provides a mobile app for logging symptoms, managing wearables, and communicating with trial teams, simplifying parental responsibilities. - What challenges do parents face in pediatric trials?
Parents often struggle with balancing responsibilities, understanding medical data, and managing emotional stress during the trial. - How do wearables benefit pediatric clinical trials?
Wearable devices like the Apple Watch and Fitbit allow for continuous health monitoring, reducing the need for frequent clinic visits and providing real-time data to researchers. - How can parents improve communication with researchers?
By staying informed, promptly reporting changes, and utilizing platforms like Clinical StudyPal for real-time communication, parents can help improve trial outcomes.