Introduction
Pediatric clinical trials play a vital role in developing safe and effective treatments for children. These trials face unique challenges, such as data collection complexities and managing transitions from pediatric to adult care during long-term studies. With advances in technology, platforms like Delve Health’s Clinical StudyPal are simplifying data collection and patient management, while tools like wearable devices ensure continuous monitoring. This article will delve into how pediatric clinical trials can overcome these challenges by enhancing data collection processes and smoothly navigating transitions.
In this article, we will explore the challenges of pediatric clinical trials, how technology can improve data collection, strategies for navigating patient transitions from pediatric to adult care, and the role of wearable devices in pediatric studies. Later, we’ll look at how specific platforms like Clinical StudyPal from Delve Health are addressing these needs with innovative solutions.
The Unique Challenges of Pediatric Clinical Trials
Pediatric clinical trials are inherently more complex than adult trials, due to the physiological, developmental, and psychological differences between children and adults. Children’s bodies react to medications differently, and their growth and development must be considered throughout the trial.
Key challenges in pediatric trials include:
- Recruitment and retention: Parents and guardians may be hesitant to enroll their children in clinical trials, often due to concerns about safety. Once enrolled, retaining young participants can be challenging due to lifestyle changes or health improvements.
- Ethical considerations: Since children cannot provide informed consent, researchers must work closely with parents or guardians, adding layers of ethical oversight.
- Data collection: Collecting accurate, timely data from children—especially young or very sick patients—can be difficult. This makes real-time, non-invasive data collection solutions particularly valuable.
How Technology Improves Data Collection in Pediatric Trials
Advances in technology, particularly with remote monitoring and wearables, have made data collection more seamless in pediatric trials. Devices like the Apple Watch and Fitbit are used to gather real-time data on heart rate, sleep patterns, and physical activity, ensuring that researchers get accurate information without requiring frequent clinic visits. Read more about the impact of wearables in clinical trials at ClinicalTrials.gov.
Key technological solutions include:
Remote Monitoring: Platforms that offer remote data collection allow researchers to track vital signs, activity levels, and other health metrics without requiring the child to leave home. This helps in collecting continuous, reliable data and reduces the burden on families.
Digital Data Capture: Electronic data capture systems enable more accurate and faster reporting. Parents can easily log symptoms, medication schedules, or adverse events through mobile apps, allowing for more precise data collection in real time.
Wearable Devices: Wearables such as fitness trackers and health monitoring patches allow non-invasive tracking of vital signs and physical activity. This is especially useful in pediatric trials where frequent hospital visits for data collection might be difficult or distressing for the child.
Navigating Patient Transitions from Pediatric to Adult Care
One of the more unique challenges in pediatric clinical trials is managing the transition when patients reach adulthood mid-study. As children grow and their physiological needs change, treatment protocols must adapt. Tools like Clinical StudyPal provide flexible solutions for managing these transitions, including real-time data adjustments and digital consent forms for patients who turn 18. Learn more about the importance of pediatric clinical trials at the FDA.
Key strategies for managing these transitions include:
Protocol Adjustments: Clinical trial protocols must be flexible enough to adapt to the changing needs of patients as they grow older. For example, changes in metabolism, dosage requirements, and physiological responses might need to be addressed.
Re-Consent Process: When a pediatric patient turns 18, they must legally re-consent to remain in the trial as an adult. Ensuring a seamless process for obtaining adult consent is critical to maintaining compliance without disrupting the study.
Consistent Data Collection: As patients transition from pediatric to adult care, it’s important that data collection remains consistent. Using tools like wearables, which track data continuously, can ensure uninterrupted data flow, even as care protocols evolve.
Introducing Delve Health’s Clinical StudyPal: An Innovative Solution
As the complexities of pediatric clinical trials continue to evolve, platforms like Delve Health’s Clinical StudyPal are emerging as powerful tools for researchers. Clinical StudyPal offers comprehensive solutions to the challenges discussed earlier, helping optimize data collection, streamline patient management, and support transitions during trials.
Key ways Clinical StudyPal enhances pediatric clinical trials:
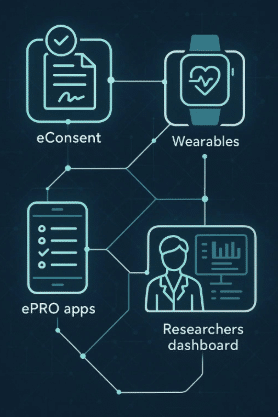
Patient-Centric Mobile App: Clinical StudyPal provides an intuitive mobile platform that allows both pediatric patients and their caregivers to engage with the trial from home. Through the app, caregivers can log symptoms, monitor medication use, and report adverse events, all while maintaining consistent communication with trial coordinators.
Remote Monitoring and Wearables Integration: Clinical StudyPal seamlessly integrates with wearable devices, enabling continuous monitoring of vital signs like heart rate, physical activity, and sleep patterns. This real-time data collection reduces the need for in-person visits while improving the overall quality of the data.
Transition Management Tools: For trials that span both pediatric and adult care, Clinical StudyPal offers tools that make it easier to transition patients. These include digital consent forms for adult patients and flexible data collection protocols that adjust to the changing needs of patients as they age.
Most Common Wearables Used in Pediatric Clinical Trials
Wearable devices have become an invaluable asset in pediatric clinical trials due to their ability to provide continuous, non-invasive data. These devices reduce the need for frequent clinical visits and ensure that researchers receive real-time, accurate data.
Here are some of the most commonly used wearables in pediatric trials:
Apple Watch: Increasingly used in pediatric trials, the Apple Watch offers a range of health tracking features including heart rate monitoring, ECG capabilities, and physical activity tracking. Its integration with health apps makes it a valuable tool for collecting data on cardiovascular and fitness-related metrics.
Fitbit: Popular for tracking physical activity, heart rate, and sleep, Fitbit devices are commonly used in pediatric studies to monitor daily activity levels and overall health.
ActiGraph: ActiGraph devices are frequently used to monitor physical activity and sleep patterns. They are often employed in trials related to ADHD, obesity, or behavioral disorders.
Oura Ring: This small, ring-shaped wearable tracks body temperature, heart rate variability, and sleep quality. Its non-intrusive design makes it a great option for pediatric patients who may not tolerate bulkier wearables.
Patch-Based Sensors (BioTelemetry): These small, adhesive patches are used to monitor vital signs such as heart rate, respiration, and glucose levels. They are particularly useful for younger pediatric patients who may find wrist-based wearables uncomfortable.
Spirometry Wearables: Devices like the MIR Spirodoc monitor respiratory functions, making them valuable in pediatric trials focused on asthma, cystic fibrosis, or other lung-related conditions.
Conclusion
Pediatric clinical trials are crucial for developing treatments that are safe and effective for children, but they come with unique challenges. Technology, especially tools like Delve Health’s Clinical StudyPal, is helping to address these challenges by improving data collection, simplifying patient management, and supporting transitions from pediatric to adult care.
As wearable devices continue to evolve, they will play an increasingly significant role in pediatric trials, offering non-invasive, real-time data collection that benefits both patients and researchers.
FAQs
What are the biggest challenges in pediatric clinical trials? Recruitment, ethical considerations, and data collection are among the most difficult aspects of pediatric trials due to the unique needs of children.
How does technology improve pediatric trials? Remote monitoring, wearables, and platforms like Clinical StudyPal allow for more accurate, real-time data collection, reducing the need for frequent hospital visits and improving patient engagement.
What happens when pediatric patients transition to adult care during a trial? Adjustments to treatment protocols and consent processes must be made when patients turn 18, and tools like Clinical StudyPal make this transition smoother by offering flexible data collection and re-consent options.
What are some of the most commonly used wearables in pediatric trials? Popular wearables include the Apple Watch, Fitbit, ActiGraph, Oura Ring, patch-based sensors, and spirometry devices, which allow for non-invasive, continuous monitoring.
How does Clinical StudyPal support pediatric trials? Clinical StudyPal enhances data collection through remote monitoring, wearable integration, and user-friendly mobile apps, ensuring that pediatric trials are more efficient and patient-friendly.
Internal Links for Further Reading: