SERVICES
ACADEMIC RESEARCH
Accelerate Your Academic Research — Digitally.
A decentralized trial platform built for researchers — compliant, multilingual, and budget-friendly.
Starting at $500 a month
INTEGRATED WEARABLES





| Feature | Included in Base Plan | Optional Add-on |
|---|---|---|
| IRB-ready eConsent (21 CFR Part 11) | ✅ | — |
| ePRO/eCOA collection (patient surveys) | ✅ | — |
| Delve EDC for structured data capture | ✅ | — |
| Delve eTMF for document management | ✅ | — |
| BYOD-friendly access | ✅ | — |
| Real-time site and PI dashboards | ✅ | — |
| Multilingual AI engagement tools (65+ languages) | ✅ | — |
| BLE and Cellular Wearable Integration | — | ✅ ($99/device + $75/patient) |
| Dedicated academic support team | ✅ | — |
| Concierge service (for complex protocols) | — | ✅ ($100/patient) |
HEAR FROM THE RESEARCH COMMUNITY
TRANSPARENT PRICING TIER
Explore our academic pricing options designed to match your study size and budget.
| Patients | Core Platform (eConsent, ePRO, EDC, eTMF) | With Wearables |
| Up to 50 | $500/month | $1,250/month |
| Up to 100 | $750/month | $1,750/month |
| Up to 250 | $1,000/month | $2,500/month |
| 500+ | Contact us for a custom quote | Contact us |
WHY RESEARCHERS CHOOSE DELVE
CHALLENGES AND CONSIDERATIONS
- Deploy in 2–4 weeks, not months
- Everything you need for IRB and compliance
- Designed for NIH, PCORI, and FDA-aligned protocols
- Flexible enough for global or community-based trials
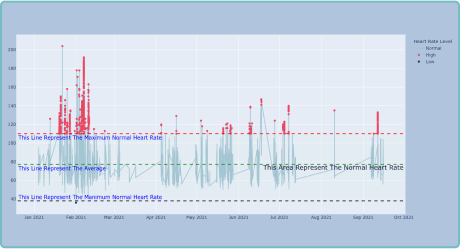
Benefits of DELVE Wearables Capabilities
- Continuous Monitoring: Provides real-time insights into patient health, allowing for timely interventions.
- Enhanced Data Accuracy: Reduces recall bias and offers objective measurements.
- Improved Patient Engagement: Empowers patients to actively participate in their health management.
- Accelerated Drug Development: Facilitates more efficient and effective clinical trials, potentially reducing time to market.
The Academic Essentials base plan includes 1 protocol, up to 50 patients, IRB-ready eConsent, ePRO/eCOA, Delve EDC, eTMF, real-time dashboards, and multilingual AI engagement tools. No hidden fees.
Yes. Delve Health is built to support federally funded studies and aligns with NIH, PCORI, and FDA guidance on digital tools, real-world data, and remote monitoring.
Yes. Patients can use their own mobile devices to complete eConsent, ePROs, and receive study-related notifications — reducing burden and increasing compliance.
We provide an IRB toolkit with pre-filled protocol language, security documentation, and regulatory references to streamline IRB submission and approval.
Yes. Wearables are optional and can be added anytime for $99/device + $75/patient for monitoring. Cellular-enabled options are available if patients don’t have smartphones.
Yes. We support integration with REDCap, OpenClinica, and other systems through secure APIs. EDC exports are also compatible with most university data platforms.
Absolutely. We provide budget templates and scope-of-work language to insert into NIH, NSF, and private foundation proposals. Our team can help you get started.
