SERVICES
Decentralized Clinical Trials
Clinical trials that meet patients where they are.
Decentralized Clinical trials with a Focus on Retention & Compliance
Delve Health delivers truly patient-centric decentralized and hybrid trials through AI-powered pre-screening, multilingual ePROs, connected wearables, and automated patient engagement. Our goal: improve retention, boost compliance, and simplify the experience—for both patients and sites.
Why Sponsors Choose Delve Health for Decentralized Clinical Trials
Delve Health is more than just a decentralized clinical trial vendor.
We’re a full-service DCT platform provider, built to meet the complex needs of modern clinical research. Sponsors choose us to accelerate study startup, boost patient retention, and ensure compliance—across hybrid and fully decentralized clinical trials.

Unified, End-to-End Platform
Avoid the inefficiencies of disconnected vendors. Delve Health integrates ePRO, eConsent, wearable data, and patient engagement into one seamless DCT platform.
Rapid Study Deployment
Launch decentralized or hybrid trials in days, not months. Our team handles device provisioning, logistics, and onboarding so you can focus on results.
Global Patient Access & Multilingual Support
Reach underserved populations with multilingual ePROs, 65+ language support, and AI agents that automate reminders and patient interactions globally.
AI-Powered Automation
From pre-screening to engagement nudges, our AI agents reduce site burden, prevent protocol deviations, and keep patients compliant throughout the study.
Regulatory-Grade Security & Compliance
We’re certified to the highest data security and compliance standards: ISO/IEC 27001, HITRUST CSF, and SOC 2. Your patient data is safe, compliant, and always accessible.
Proven Patient Engagement
Our digital engagement engine, supported by 32 AI agents and concierge services, delivers over 90% patient compliance—even in long-term and complex studies.

Delve Health transforms decentralized clinical trials into compliant, scalable, and patient-centric experiences—designed for today’s sponsors.
LET'S TALK
Benefits of Running Decentralized Clinical Trials with Delve Health
Decentralized clinical trials (DCTs) are transforming the way research is conducted—making studies more patient-centric, accessible, and efficient. Delve Health’s full-service DCT platform combines technology, automation, and human support to deliver measurable benefits across the entire clinical trial lifecycle.
Broader Patient Access and Diversity
By removing geographic and transportation barriers, DCTs enable access to more diverse and representative patient populations—including those in rural, underserved, and global communities.
Faster Recruitment and Enrollment
Delve Health’s AI-powered pre-screening and remote onboarding tools accelerate patient matching and reduce enrollment delays—cutting startup timelines significantly.
Higher Patient Engagement & Retention
Our 32 AI agents and multilingual concierge team keep patients engaged throughout the trial via personalized reminders, automated nudges, and real-time support—resulting in fewer dropouts and greater protocol adherence.
Reduced Site Burden and Operational Overhead
Decentralized models shift tasks from sites to the platform—freeing up staff from repetitive duties like reminder calls, visit scheduling, and tech troubleshooting. This leads to lower burnout and increased study efficiency.
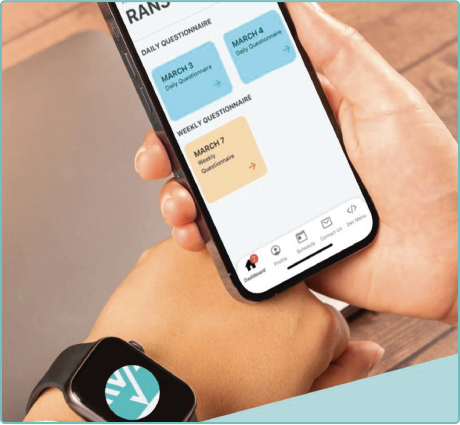
Real-Time Data Collection & Insights
Using FDA-grade wearables and digital assessments, Delve Health captures continuous, real-time data remotely—providing sponsors with faster, cleaner insights for faster decision-making.
Cost Savings and Better ROI
Decentralized trials reduce the need for physical sites, on-site monitoring, and in-person visits—lowering operational costs while maintaining data quality and improving study timelines.
Real-World Data and Analytics
Our Clinical StudyPal platform is designed to connect higher quality real-world data (RWD) and provide robust analytics. This capability helps sites solve challenges and develop more diverse, patient-centric clinical trials. By leveraging these insights, clinical trials can be tailored to meet the needs of diverse patient populations, improving overall trial outcomes. The platform’s analytics capabilities provide valuable data that can be used to refine trial procedures, enhance patient engagement strategies, and improve overall trial efficiency.

Why Choose Delve Health for Patient Concierge Services?
Expertise in
Clinical Trials
Our team comprises clinical trial experts who provide tailored patient concierge services to meet the specific needs of each trial, ensuring optimal outcomes.
Advanced Technology Support
The Clinical StudyPal platform automates routine tasks, provides real-time analytics, and ensures seamless patient communication, enhancing efficiency and trial outcomes.
Proven Track
Record
Delve Health has a successful history of supporting clinical trials across various therapeutic areas and phases, consistently improving patient outcomes and trial efficiency.
Frequently Asked Questions
A decentralized clinical trial (DCT) is a type of clinical study that uses digital health technologies to reduce or eliminate the need for in-person visits. DCTs enable remote patient participation through tools like eConsent, ePROs, wearable devices, telemedicine, and digital engagement platforms.
Decentralized trials offer multiple advantages, including:
Faster patient recruitment and broader geographic reach
Increased patient retention through remote engagement
Real-time data collection via wearable sensors and digital diaries
Reduced site burden and operational costs
Greater diversity and inclusion in trial populations
Delve Health provides a unified DCT platform—Clinical StudyPal—that integrates:
AI-powered pre-screening
Multilingual eConsent and ePROs
FDA-grade wearable data collection
Automated patient engagement via 32 AI agents
Human concierge services for global patient support
All services are built to support hybrid or fully remote clinical trial models.
Yes, DCTs can be applied across Phase I to Phase IV trials, though implementation strategies may vary. Delve Health customizes each solution to fit the study’s complexity, patient population, and regulatory requirements.
In many cases, yes. Decentralized trials can reduce costs related to:
Site operations and overhead
On-site monitoring and travel
Missed visits and patient dropout
Manual data entry and protocol deviations
The result is improved ROI without compromising data quality.
DCTs enhance compliance by making trial participation more convenient. With automated reminders, mobile-friendly tools, and 24/7 support, patients are more likely to stay on track—leading to cleaner, more complete data.
Absolutely. We specialize in mid-study intervention, offering DCT rescue services that improve retention, streamline logistics, and automate patient engagement—without disrupting ongoing operations.




























