PLATFORM
Patient
engagement
Transforming Patient Engagement in Clinical Trials
At Delve Health, we understand that patient engagement is the cornerstone of successful decentralized clinical trials. Our Clinical StudyPal platform is designed to enhance patient experience, increase compliance, and improve retention through innovative digital solutions.

Why Patient Engagement Matters
Engaging patients effectively leads to higher trial retention, better data quality, and improved study outcomes. Traditional clinical trials often suffer from low adherence due to complex protocols, travel burdens, and lack of real-time interaction. Our patient-first approach removes these barriers by leveraging:
✅ Wearable Devices & Digital Biomarkers – Seamlessly track real-time health data without disrupting daily life.
✅ AI-Driven Engagement Automation – Personalized reminders, nudges, and check-ins improve adherence.
✅ Multilingual eConsent & Digital Education – Ensure patients understand the trial in their preferred language.
✅ Gamification & Reward Systems – Encourage participation through interactive and motivational strategies.
✅ Telehealth & Remote Monitoring – Enable continuous engagement without in-person site visits.
How Delve Health Enhances Patient Engagement
Unparalleled Data
Collection
1. Personalized & Multilingual Communication
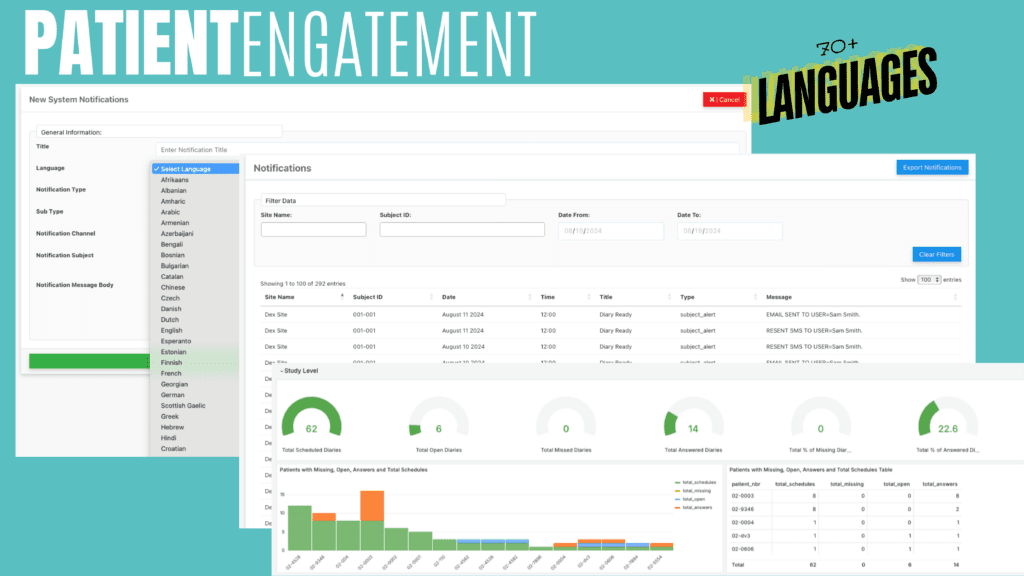
Patients receive customized notifications, educational materials, and engagement prompts in 65+ languages, ensuring inclusivity and accessibility.
2. Real-Time Data & Automated Reminders
Our system integrates with wearables, apps, and remote sensors, providing researchers with real-time patient data while sending automated adherence reminders.
3. Flexible Participation with Digital & Hybrid Models
Whether fully decentralized or hybrid, Delve Health ensures seamless interaction between patients and research teams, reducing drop-out rates.
4. AI & Behavioral Insights for Continuous Improvement
Using AI-driven analytics, researchers can identify engagement trends and adjust protocols to enhance participation and retention.
Proven Results in Patient-Centered Research
✔ Higher Retention – Studies show that engaged patients are more likely to complete trials.
✔ Improved Data Quality – Consistent engagement leads to more reliable real-world evidence.
✔ Reduced Burden on Patients – Less travel, more flexibility, and better study experiences.
Enhancing retention, compliance, and data quality with patient-first technology.
Frequently Asked Questions
Patient engagement refers to the strategies and technologies used to keep participants informed, involved, and motivated throughout the clinical trial process. Delve Health enhances engagement with AI-driven automation, wearables, multilingual eConsent, and digital tools that simplify participation.
We use personalized communication, automated reminders, gamification, and telehealth integration to keep patients engaged and reduce dropout rates. Our solutions provide flexibility and convenience, allowing participants to contribute without the burden of frequent in-person visits.
Delve Health’s platform integrates with a wide range of wearable devices, biosensors, and digital biomarkers to collect real-time patient data, ensuring seamless remote monitoring and improved trial outcomes.
Our eConsent solution supports 65+ languages, providing participants with clear, culturally appropriate digital consent forms, multimedia explanations, and educational materials to ensure informed participation.
Yes! We support both fully decentralized and hybrid trial models, allowing for a mix of remote and in-person interactions, ensuring flexibility for researchers and participants.
Our AI-driven platform analyzes patient behaviors, engagement patterns, and compliance rates to deliver personalized reminders, nudges, and adaptive interventions, improving adherence and study success.
Remote monitoring reduces site visits, minimizes patient burden, and provides real-time insights into patient health, enabling early intervention and more accurate trial data collection.
Yes! We ensure HIPAA, GDPR, and FDA compliance, prioritizing patient data security and privacy in all aspects of decentralized clinical trials.
Our solutions help increase retention, improve data quality, streamline workflows, and reduce trial costs, ultimately leading to more efficient, patient-centric clinical trials.
