SERVICES
Post-Market Clinical Studies
Clinical trials that meet patients where they are.
Connect with Us
Real-World Evidence That Goes Beyond Approval
Delve Health supports sponsors in designing and executing post-market studies that capture real-world data (RWD), demonstrate ongoing safety and effectiveness, and ensure long-term patient engagement. Our platform enables scalable, cost-effective post-marketing surveillance with the same innovation and automation that power our decentralized trials.
Why Sponsors Choose Delve Health for Post-Market Studies
Post-market studies require a different approach—focused on long-term outcomes, real-world data collection, and patient retention over time. Delve Health is uniquely positioned to help sponsors collect meaningful data while keeping patients engaged and sites efficient.
Supporting Long-Term Evidence Generation and Compliance

-
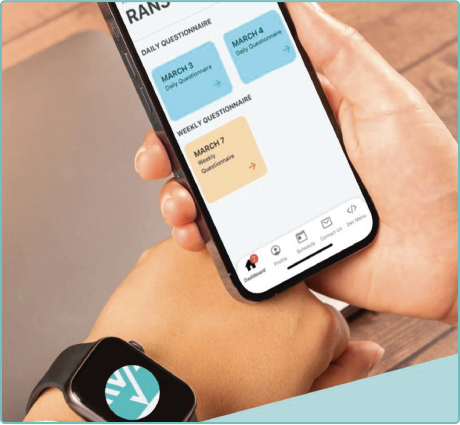
Unified Platform for Real-World Data Collection
Seamlessly capture data through ePROs, wearables, patient diaries, and automated follow-ups in one streamlined environment. -
Rapid Study Deployment
Quickly launch post-market studies to monitor products post-approval and meet regulatory expectations without delays. Whether you’re running a PMCF, registry or RWE initiative-without needing a CRO or internal tech build. -
Global Scalability & Multilingual Support
Support international post-market programs with tools available in 65+ languages and remote monitoring capabilities.
AI-Powered Patient Engagement
Keep patients active in the study for months or years with AI-driven reminders, nudges, and real-time alerts for potential non-compliance.Secure & Compliant Platform
Meet requirements for FDA, EMA, and other global regulators with full data privacy controls (ISO/IEC 27001, HITRUST CSF, SOC 2).Long-Term Patient Retention
Engagement strategies that maintain participation through concierge services, gamification, and patient-centric tools.

Delve Health transforms decentralized clinical trials into compliant, scalable, and patient-centric experiences—designed for today’s sponsors.
LET'S TALK
Benefits of Running Post-Market Studies with Delve Health
Post-approval studies require sustained commitment. Delve Health brings modern digital tools and operational support to deliver better compliance, real-time data, and actionable insights.
Real-World Evidence & Longitudinal Data
Collect continuous real-world data (RWD) on safety, efficacy, and product usage in routine clinical settings.
Improved Regulatory Compliance
Our eConsent, ePROs, and audit-ready platform streamline documentation and compliance with FDA, EMA, and PMCF requirements.
Greater Patient Participation
Patients can participate remotely, reducing dropouts and making studies more accessible across diverse geographies and age groups.
Reduced Operational Complexity
Automated follow-ups, wearable logistics, and multilingual support reduce site burden and simplify execution.
Enhanced Data Quality
Real-time alerts and engagement automation improve data completeness, protocol adherence, and monitoring.
Flexible Study Design
Delve Health supports a range of post-market study types, including: Phase IV safety and effectiveness follow-up, Medical device Post-Market Clinical Follow-Up (PMCF), Registries and observational cohorts, and Patient-reported outcome tracking
Real-World Data and Analytics
Our Clinical StudyPal platform is designed to connect higher quality real-world data (RWD) and provide robust analytics. This capability helps sites solve challenges and develop more diverse, patient-centric clinical trials. By leveraging these insights, clinical trials can be tailored to meet the needs of diverse patient populations, improving overall trial outcomes. The platform’s analytics capabilities provide valuable data that can be used to refine trial procedures, enhance patient engagement strategies, and improve overall trial efficiency.

Why Choose Delve Health for Post-Market Study Support
Expertise in Real-World Studies
We understand the complexity of long-term follow-up, post-approval mandates, and stakeholder expectations.
Platform Built for Engagement and Evidence
Clinical StudyPal automates communication, captures wearable and ePRO data, and adapts to your protocol needs over time.
Proven Outcomes
Delve Health has successfully supported global post-market and registry studies with measurable improvements in retention, data quality, and reporting speed.
Frequently Asked Questions
A post-market study collects real-world data after a product has received regulatory approval to assess long-term safety, effectiveness, or real-world use patterns.
They provide ongoing surveillance, inform future labeling or clinical decisions, and fulfill regulatory obligations in real-world settings.
Our platform includes wearables, ePROs, eConsent, digital diaries, AI engagement agents, and global concierge support—all integrated in a unified dashboard.
Yes. Delve Health enables fully remote or hybrid post-market trials with digital tools that reduce the need for physical site visits.
Yes. We meet global standards including ISO/IEC 27001, HITRUST CSF, SOC 2, and support regulatory-ready data exports.
Absolutely. We support device monitoring, symptom tracking, and long-term outcome studies with remote data capture.
