PLATFORM
Wearables in Clinical Trials
The Role of Wearables in Clinical Trials
Integrating wearable devices into clinical trials has revolutionized data collection, patient engagement, and overall study efficiency. At Delve Health, we leverage advanced wearable technology to enhance decentralized clinical trials, ensuring comprehensive, real-time insights into patient health.
Wearable devices, encompassing sensors and software applications on smartphones and tablets, enable the remote collection of health-related data. This capability allows for continuous monitoring of patients outside traditional clinical settings, providing a more holistic view of patient health and behavior.

Benefits of Integrating Wearables
Continuous Data Collection: Wearables facilitate 24/7 data gathering, offering a comprehensive view of patient health metrics, including activity levels, heart rate, and sleep patterns.
Enhanced Patient Engagement: By reducing the need for frequent clinic visits, wearables minimize patient burden, leading to improved participation and retention rates in clinical trials.
Real-World Data Acquisition: Wearables provide longitudinal biometric data, offering unique insights into the long-term, real-world impact of treatments and interventions.
How Delve Health LEVERAGES WEARABLES IN CLINICAL TRIALS
Challenges and Considerations
While wearables offer significant advantages, several challenges must be addressed:
Data Validation: Ensuring the accuracy and reliability of data collected from various wearable devices is crucial.
Data Management: Processing and analyzing the vast amounts of data generated by wearables require robust infrastructure and analytical tools.
Privacy and Security: Protecting patient data and maintaining compliance with regulatory standards are paramount.
Addressing these challenges involves selecting appropriate devices, establishing clear data collection protocols, and implementing stringent data security measures.
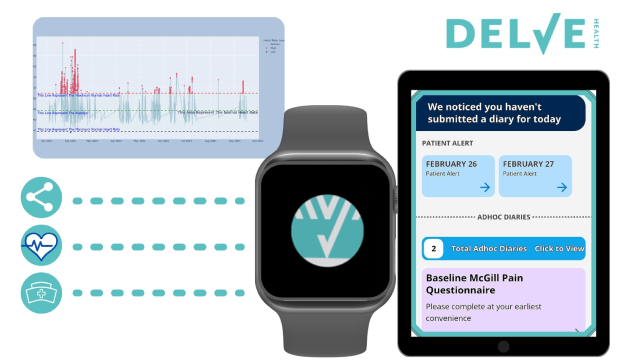
Delve Health's Approach
At Delve Health, we integrate wearable technology into our decentralized clinical trial platform to:
Seamless Data Integration: Our platform supports various wearable devices, ensuring smooth data collection and real-time monitoring.
Patient-Centric Design: We prioritize user-friendly interfaces and patient engagement tools to enhance compliance and retention.
Advanced Analytics: Utilizing AI-driven analytics, we transform raw data into actionable insights, facilitating informed decision-making in clinical trials.
Device Portfolio
| Device | Key Capabilities / Biomarkers | Therapeutic Areas of Focus | Regulatory Approval | What This Replaces / Why This Is Better | Why Use This Device in Research | Delve Health's Expertise | Relevance to Rare Diseases & Other Therapeutic Areas |
|---|---|---|---|---|---|---|---|
| Dexcom CGM | Continuous glucose monitoring (CGM); Real-time alerts | Diabetes (Type 1 & 2); Hyper/hypoglycemia management | FDA-cleared for CGM in diabetes | Replaces manual fingerstick glucose checks; Better for 24/7 dynamic data and proactive insulin titration | High-frequency glucose data for glycemic variability; Real-time insights to guide insulin therapy | Integrated Dexcom in decentralized diabetes trials; Delivers 24/7 remote monitoring and patient engagement | Rare metabolic or monogenic diabetes (MODY); Congenital hyperinsulinism; CF-related diabetes |
Ametris (formerly Actigraph) | Activity levels (movement); Sleep-wake cycles | Sleep disorders; General wellness (fitness, mobility) | Widely validated in research (used in academia and clinical studies) | Replaces subjective diaries for sleep and activity; Better for continuous, objective measurement | Gold-standard for objective sleep and activity data; Trusted in peer-reviewed studies | Experience in large-scale observational studies using Actigraph for sleep/activity endpoints | Ideal for neurological or movement-based rare diseases (e.g., Rett syndrome, Fragile X, muscular dystrophies) |
| Apple Watch | Heart rate (HR); ECG (Series 4+); Steps/Activity; Basic sleep tracking | Cardiac arrhythmias (A-fib); Cardiovascular health; Wellness | FDA De Novo clearance for ECG (U.S., Series 4+) | Replaces separate Holter or event monitors (milder cases); Better for seamless, consumer-friendly data capture and high adoption | Large consumer adoption improves compliance; FDA-cleared ECG aids arrhythmia detection | Integrates Apple HealthKit data into Delve’s platform; Proven in cardiac and wellness population studies | Inherited arrhythmic disorders (e.g., Long QT, Brugada); Connective tissue disorders (e.g., Ehlers-Danlos) for continuous monitoring |
| Fitbit | Heart rate; Steps/Activity; Sleep stages; SpO2 (select models) | General fitness and wellness; Weight management; Cardiovascular | Some models (e.g., Sense) FDA-cleared for ECG in select regions | Replaces traditional pedometers and subjective activity logs; Better for affordable, scalable, multi-metric tracking | Cost-effective, mass-market wearables; Large user base for broad recruitment | Seamless API integration into Delve’s workflows; Proven in population-level remote monitoring | Useful in pulmonary hypertension, rare anemias, or any rare disease with fatigue or sleep components to track daily function |
| Oura Ring | Heart rate (HR); Heart rate variability (HRV); Sleep stages; Body temperature; Respiratory rate | Stress and recovery; Sleep disorders; Early illness detection | Consumer wellness device (not FDA-cleared); Used in validated research collaborations | Replaces cumbersome overnight polysomnography (for basic data); Better for unobtrusive ring-based continuous tracking | Non-intrusive ring with advanced sleep and recovery metrics; Ideal for longitudinal remote monitoring | Deployed in stress, sleep, and recovery studies; Correlates Oura metrics with clinical endpoints | Potential for autoimmune or neurodegenerative rare diseases (e.g., capturing subtle changes in HRV, temperature, sleep) |
| Samsung Watch | Heart rate; ECG (select models); Steps/Activity; Sleep; Blood pressure (in some regions) | Hypertension; Cardiac arrhythmias; Fitness and wellness | FDA-cleared ECG in the U.S.; BP monitoring approved in select countries | Replaces manual BP cuffs and separate ECG devices (select models); Better for integrated vitals in a large Android user base | Large Android ecosystem; Potential for blood pressure tracking in certain markets | Expertise in global, multi-country research; Integrations with Android for broad patient reach | Inherited hypertension syndromes, Marfan syndrome, or other conditions with cardiovascular involvement (activity, BP, ECG) |
| Circul+ | Heart rate; SpO2; Respiratory rate; Sleep tracking; Possible BP | Respiratory conditions; Cardiovascular monitoring; Sleep disorders | CE Mark in some regions; (FDA status may vary by model) | Replaces clinic-based overnight oximetry; Better for ring-based continuous SpO2, HR, and respiratory data | Ring-like wearable for 24/7 HR & SpO2 monitoring; Ideal for continuous sleep and cardiorespiratory research | Delve supports multivariable data in decentralized settings; Great for overnight or post-discharge monitoring | Useful in cystic fibrosis, pulmonary fibrosis, or other rare lung diseases requiring high-frequency SpO2 and HR monitoring |
| Spirolink | Lung function (FEV1, FVC, etc.) | Asthma; COPD; Other pulmonary diseases | Typically FDA/CE-cleared for spirometry | Replaces in-clinic spirometry visits; Better for at-home measurements (reduces travel, increases test frequency) | Portable spirometry for at-home pulmonary function testing; Key endpoint in respiratory trials | Specialized in respiratory trial management; Integrates FEV1 data with symptom tracking and telehealth | Applicable to alpha-1 antitrypsin deficiency, cystic fibrosis, idiopathic pulmonary fibrosis, or other rare lung conditions |
| Strados | Respiratory acoustics (lung sounds); Respiratory rate | Asthma; COPD; Chronic respiratory monitoring | FDA 510(k) clearance for acoustic respiratory monitoring | Replaces stethoscope-based spot checks in clinic; Better for real-time detection of wheezes, coughs, and exacerbations | Real-time detection of wheezes/coughs; Alerts for exacerbation events | Advanced analytics for exacerbation detection; Used in remote pulmonary monitoring programs | Could help in bronchiectasis, primary ciliary dyskinesia, or other rare respiratory disorders with unique lung-sound patterns |
| Withings | Weight, Body Composition; Blood Pressure; Heart rate; ECG (some devices) | Hypertension; Obesity and weight management; Arrhythmias | Some devices FDA/CE-cleared (e.g., BPM Core, ScanWatch) | Replaces standalone BP cuffs, scales, and separate ECG devices; Better for consolidated multi-endpoint tracking | Multi-device ecosystem (scales, BP monitors, watches) offering multiple biometric endpoints in one platform | Orchestrates holistic health tracking (weight, BP, ECG) in Delve’s decentralized trials; Longitudinal data capture | Helps monitor weight and BP in rare metabolic syndromes (lipodystrophy); Tracks autonomic dysfunction in certain rare conditions (e.g., POTS) |
Alivecor Kardia | ECG: 1-lead, 6-lead, and 12-lead; AI-based arrhythmia detection | Atrial fibrillation; Other arrhythmias | FDA-cleared portable ECG device | Replaces bulky Holter monitors or in-clinic ECGs; Better for patient convenience and on-demand diagnostic-grade ECG (6- or 12-lead) | Medical-grade ECG in a compact form; On-demand arrhythmia detection and analysis | Delve’s platform streamlines ECG data capture for cardiac trials; Facilitates remote arrhythmia monitoring | 12-lead option aids in inherited arrhythmias (e.g., Brugada, CPVT, HCM); Useful for rare cardiac channelopathies requiring advanced ECG insights |
By embracing wearable technology, Delve Health is at the forefront of transforming clinical trials, making them more efficient, patient-friendly, and data-rich.
Regulatory Compliance & Data Security
At Delve Health, we prioritize the security and privacy of patient data. Our platform ensures:
HIPAA & GDPR Compliance: Adherence to international standards for data protection and patient confidentiality.
Secure Data Management: Implementation of advanced encryption and access control measures to safeguard information.
Frequently Asked Questions
Wearable devices in clinical trials refer to sensors or software applications on smartphones and tablets that remotely collect health-related data outside traditional clinical settings.
Wearables offer continuous data collection, enhance patient engagement by reducing the need for frequent clinic visits, and provide real-world data acquisition, leading to more comprehensive insights into patient health.
Wearable devices can monitor various health metrics, including physical activity levels, heart rate, sleep patterns, and other physiological parameters, providing a comprehensive view of a participant’s health status.
Yes, challenges include ensuring data accuracy and validation, managing large volumes of data, and addressing privacy and security concerns to protect patient information.
Delve Health’s platform supports various wearable devices, ensuring seamless data collection and real-time monitoring. We prioritize user-friendly interfaces and employ advanced analytics to transform raw data into actionable insights, enhancing the efficiency and effectiveness of clinical trials.
