Delve Health offers a comprehensive platform that significantly enhances the efficiency and effectiveness of clinical trials. Our solution integrates wearable technology, Clinical Outcomes Assessments (COAs), and automated patient engagement tools, providing an end-to-end solution for researchers. Here’s a closer look at how Delve Health is transforming clinical trials, particularly in creating optimized patient workflows through a mobile app, and supporting diversity and inclusion of underrepresented populations.
Comprehensive Wearable Integration
Wearable Library: Delve Health partners with leading wearable device manufacturers to bring advanced patient measures directly to clinical trials. Our extensive library includes devices from renowned brands such as Dexcom, Strados, TempTraq, Withings, Oura, Apple, Actigraph, Sensomics, Circul+, Kardia, PKG, and Fitbit. These devices cover a wide range of health metrics, including heart rate, SPO2, sleep patterns, blood pressure, and physical activity.
Challenges Wearable Companies Face in Monitoring Wear-Compliance
Wear-Compliance Monitoring: One of the significant challenges wearable companies face is ensuring that participants consistently wear their devices. Factors contributing to this difficulty include:
- Participant Forgetfulness: Participants may forget to wear their devices regularly, leading to gaps in data collection.
- Comfort and Usability: Some wearables may be uncomfortable or inconvenient to wear for extended periods, reducing compliance.
- Technical Issues: Battery life, connectivity problems, and device malfunctions can interrupt continuous monitoring.
- Data Synchronization: Ensuring that data is correctly and timely synchronized from multiple devices can be complex and error-prone.
- Behavioral Resistance: Some participants may resist wearing the devices due to privacy concerns or skepticism about the technology.
Optimized Patient Workflow with Mobile App Integration
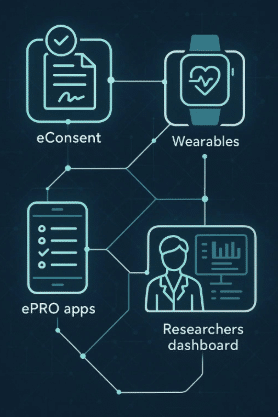
Mobile App Integration: Delve Health addresses these challenges by integrating wearable data with a user-friendly mobile app, creating an optimized patient workflow where participants know exactly what tasks need to be completed and when.
Task Management: The mobile app provides a clear, intuitive interface that outlines daily tasks, reminders, and deadlines. Patients receive automated notifications about upcoming tasks, such as wearing their devices, completing surveys, or attending virtual check-ins.
Real-Time Feedback and Support: The app offers real-time feedback on task completion and compliance, helping patients stay on track. It also includes features for instant communication with the study team, ensuring patients can easily report issues or ask questions.
Automated Engagement Tools: Delve Health employs AI-driven tools within the mobile app to automate patient engagement. This includes personalized messages, motivational prompts, and educational content, all designed to keep patients engaged and compliant.
Data Integration and Access: The app integrates data from wearables and COAs, providing patients with a comprehensive view of their health metrics and progress. This transparency helps build trust and encourages active participation.
Supporting Diversity and Inclusion
Inclusivity in Clinical Trials: Delve Health’s platform supports decentralized and hybrid studies, making it easier for patients from diverse and underrepresented populations to participate. By reducing the need for frequent site visits and enabling remote participation, our platform ensures that clinical trials are more accessible to a broader range of participants.
Addressing Barriers to Participation: The mobile app and wearable integration help overcome common barriers to participation such as geographic limitations, transportation issues, and scheduling conflicts, which are often faced by individuals in underrepresented areas.
FDA Support for Inclusivity: The FDA emphasizes the importance of including diverse populations in clinical trials. According to the FDA, “Ensuring diverse populations in clinical trials not only facilitates broader applicability of results but also enhances the understanding of the disease or medical product under study” (FDA) (FDA).
Delivered Outcomes
Increased Efficiency and Reduced Costs: By streamlining data collection and analysis, and automating patient engagement through the mobile app, Delve Health significantly increases the efficiency of clinical trials while reducing associated costs. Our platform’s ability to rapidly design and implement studies sets it apart, allowing for quicker adaptation and optimization.
Improved Patient Engagement and Retention: The mobile app’s comprehensive engagement tools ensure that patients remain involved and compliant, leading to more reliable study results and reduced dropout rates. Our focus on seamless, real-time interaction distinguishes our approach to patient engagement.
Enhanced Data Quality: With continuous access to high-quality, real-time data, researchers can make more informed decisions, ultimately improving the outcomes of clinical trials. Our commitment to data integrity and compliance ensures that the insights derived are both robust and actionable.
Supported by FDA Articles and Client Testimonies
FDA Articles: Our platform aligns with the FDA’s guidelines and recommendations for integrating digital health technologies into clinical trials. According to the FDA, “digital health technologies can improve the efficiency of clinical trials for sponsors, investigators, and other stakeholders and may increase the opportunities for individuals to participate in research and make participation more convenient” (FDA) (FDA).
Client Testimonies:
- “Using Delve Health’s platform, we saw a significant increase in patient compliance and engagement. The ability to manage tasks through the mobile app and receive real-time feedback made a tremendous difference in our trial’s success.” – Chief Medical Officer
- “Delve Health’s comprehensive approach to integrating wearables and patient-reported outcomes has streamlined our data collection processes and improved the overall quality of our clinical trials.” – Clinical Trials Innovation Officer
Delve Health’s multi-modal digital platform is not just about integrating technology; it’s about providing a holistic solution that meets the complex needs of modern clinical trials. By combining advanced wearables, validated algorithms, and AI-driven engagement tools within a mobile app, we are paving the way for a more efficient, patient-centric approach to clinical research.
Learn more about how Delve Health can support your clinical trials at delvehealth.com.