Introduction
In the dynamic landscape of clinical research, the integration of digital measures and a deep understanding of patient-reported outcomes (PROs) are reshaping how trials are conducted. This article explores the vital role of these elements in modernizing clinical research, offering insights into their impact on trial efficiency, data accuracy, and patient engagement.
The Evolution of Clinical Research
From Traditional to Digital Methods
The journey from traditional paper-based methods to advanced digital tools marks a significant shift in clinical research. This evolution has not only streamlined processes but also opened new avenues for data collection and analysis.
The Impact of Technology on Research Efficacy
Technological advancements have significantly enhanced the efficacy of clinical trials. By harnessing digital tools, researchers can gather more comprehensive and reliable data, leading to more accurate study outcomes.
Understanding Patient-Reported Outcomes (PROs)
Definition and Importance
PROs refer to the direct feedback from patients regarding their health status, treatment experiences, and overall well-being. Understanding these outcomes is crucial for tailoring treatments to patient needs.
How PROs Transform Patient Care
The incorporation of PROs into clinical research brings a patient-centric approach to trial design, ensuring that patient experiences and outcomes are at the forefront of clinical studies.
Digital Measures in Clinical Trials
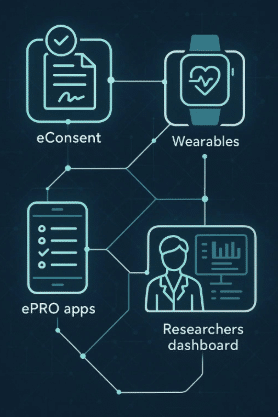
Integrating Digital Tools in Trials
The implementation of digital measures in clinical trials, such as electronic health records and mobile health apps, enhances the quality and accessibility of data.
Advantages of Digitalization in Research
Digitalization offers numerous benefits, including increased efficiency, reduced costs, and improved patient engagement, which collectively contribute to more successful clinical trials.
Enhancing Data Accuracy with Digital Measures
Precision and Reliability in Data Collection
Digital tools provide a level of precision and reliability in data collection that traditional methods cannot match, leading to more accurate and credible trial results.
Case Studies: Successes in Digital Data Accuracy
Various case studies highlight the successes achieved through digital measures in clinical trials, showcasing their impact on research accuracy and reliability.
Patient-Centric Approach in Modern Trials
The Role of PROs in Patient-Centricity
PROs play a pivotal role in adopting a patient-centric approach in clinical research, ensuring that patient experiences and feedback drive trial design and execution.
Enhancing Patient Engagement through Digital Tools
Digital tools not only facilitate data collection but also actively engage patients in their healthcare journey, leading to more informed and involved participants.
Real-Time Monitoring and Adaptive Trials
Leveraging Real-Time Data for Adaptive Research
Real-time monitoring allows for adaptive trials, where protocols can be adjusted based on ongoing data, leading to more flexible and responsive research designs.
Benefits and Challenges of Real-Time Monitoring
While real-time monitoring offers significant benefits, such as increased adaptability and timely intervention, it also presents challenges in terms of data management and analysis.
The Future of Digital Measures and PROs
Predictions and Emerging Trends
The future of clinical research lies in the further integration of digital measures and a deeper focus on PROs, with emerging trends indicating a shift towards a more digital-first approach.
Preparing for a Digital-First Research Paradigm
As the industry moves towards a digital-first paradigm, researchers and stakeholders must prepare for the changes and challenges this shift entails.
Overcoming Barriers in Digital Clinical Research
Identifying and Addressing Challenges
Despite the advantages, digital clinical research faces barriers such as technology adoption, data privacy concerns, and resistance to change, which must be addressed for successful implementation.
Strategies for Successful Implementation
Implementing digital measures in clinical research requires strategic planning, stakeholder engagement, and continuous adaptation to technological advancements.
Regulatory Considerations and Compliance
Navigating Regulatory Frameworks
Digital clinical research must navigate complex regulatory frameworks, ensuring compliance with laws and guidelines to maintain the integrity and validity of trials.
Ensuring Compliance in Digital Trials
Ensuring compliance in digital trials involves adhering to regulatory standards, maintaining data security, and ensuring ethical considerations are met.
The Economic Impact of Digitalization in Clinical Research
Cost-Efficiency and ROI
The shift to digital measures in clinical research offers cost-efficiency and a higher return on investment, benefiting stakeholders across the board.
Economic Benefits for Stakeholders
The economic benefits of digitalization extend to all stakeholders, including researchers, healthcare providers, patients, and pharmaceutical companies.
Case Study: A Breakthrough Clinical Trial
Overview of a Successful Digital Trial
An examination of a breakthrough digital trial provides insights into the practical application of digital measures and PROs in clinical research.
Lessons Learned and Best Practices
This case study offers valuable lessons and best practices for implementing digital measures and focusing on PROs in future clinical trials.
The Role of Artificial Intelligence and Machine Learning
AI in Enhancing Clinical Research
Artificial intelligence (AI) and machine learning are poised to play a significant role in enhancing clinical research, particularly in data analysis and predictive modeling.
Future Implications of AI in Patient-Reported Outcomes
The future implications of AI in the context of PROs are vast, with potential for improved patient engagement, personalized treatments, and advanced data analysis techniques.
Conclusion
The integration of digital measures and a focus on patient-reported outcomes are revolutionizing the field of clinical research. By embracing these advancements, the industry can look forward to more efficient, accurate, and patient-centered trials, ultimately leading to better health outcomes and innovations in medical research.
FAQ
- How do digital measures improve the accuracy of clinical trials?
- What are the main advantages of using patient-reported outcomes in clinical research?
- How does real-time monitoring impact the adaptability of clinical trials?
- What challenges do clinical researchers face in implementing digital measures?
- How is artificial intelligence shaping the future of clinical research?
- What role do regulatory bodies play in digital clinical research?