Introduction: How Wearables Are Transforming Clinical Trials
Clinical trials have always been the backbone of medical innovation. From testing new drugs to evaluating breakthrough therapies, clinical trials help ensure that treatments are safe and effective. But the way we conduct these trials is changing. Thanks to rapid advancements in technology, wearable devices are now playing a key role in how researchers collect data, engage patients, and run studies. Wearables in clinical trials are no longer just a trend. They are becoming a core part of how studies are designed and executed. In this blog, we’ll explore how wearable devices are transforming clinical research, why digital biomarkers in healthcare are gaining attention, and what this all means for trial sponsors, clinical researchers, and the future of medicine. Let’s take a closer look at the evolution of clinical trials and how wearable technology is making research smarter, faster, and more inclusive.What Are Wearables in Clinical Trials?
Wearables are electronic devices that people can wear on their bodies to collect and transmit health-related data. In clinical trials, these devices help researchers monitor participants in real time without needing constant clinic visits. Some common examples include smartwatches, fitness trackers, biosensor patches, and even connected clothing. These devices can track various health metrics such as heart rate, physical activity, blood oxygen levels, sleep patterns, glucose levels, and more. The beauty of wearables lies in their ability to continuously gather data in natural, everyday settings. This leads to a more accurate picture of a participant’s health compared to snapshot data from occasional doctor visits. The use of wearables in clinical trials started gaining traction over the past decade. What was once seen as experimental is now considered a valuable tool in modern research. The growing demand for real-world data and decentralized trial models has only accelerated this shift. Wearables help bridge the gap between clinical environments and daily life. They not only support data collection but also improve patient comfort and engagement throughout the study process.The Rise of Digital Biomarkers in Healthcare
A digital biomarker is a physiological or behavioral data point collected through digital devices like wearables. Unlike traditional biomarkers that often rely on lab tests or imaging, digital biomarkers are gathered passively and continuously. They offer a new way to track health changes over time in a non-invasive, scalable manner. For instance, a smartwatch that monitors heart rate variability can help identify early signs of cardiovascular stress. Similarly, a wearable patch that tracks sleep cycles may reveal signs of a neurological disorder. These kinds of digital signals are already showing promise in areas like oncology, cardiology, psychiatry, and chronic disease management. Digital biomarkers are powerful because they give researchers access to high-frequency, real-time data. Instead of relying on a few data points collected in a clinic, they can observe trends and patterns over days, weeks, or even months. This leads to more informed decision-making, faster identification of side effects, and better understanding of treatment impact. Additionally, digital biomarkers can support personalized medicine. They help researchers identify which patients are responding to treatment and which aren’t, allowing for early intervention or tailored therapies. As digital biomarkers become more reliable and validated, they are likely to become a standard part of clinical trials and broader healthcare strategies.
Remote Patient Monitoring: A Paradigm Shift
Remote patient monitoring, or RPM, allows healthcare providers and researchers to keep track of a patient’s health while they are at home or on the go. This model reduces the need for in-person visits and brings clinical oversight directly to the patient’s environment. In clinical trials, RPM powered by wearables is changing the game. Instead of asking participants to visit a clinic multiple times a month, researchers can now gather data remotely. This is especially helpful for participants with mobility issues, those living in rural areas, or individuals managing chronic conditions. For example, in a trial for a diabetes medication, a continuous glucose monitor can track blood sugar levels in real time. That data is sent directly to the research team, who can monitor trends and identify issues immediately. If something looks off, they can intervene without waiting for the next appointment. Remote monitoring also improves patient safety. When something abnormal is detected, alerts can be triggered, and the care team can take prompt action. This proactive approach reduces risk and enhances the overall quality of the trial. In the era of digital health, RPM is becoming a central feature of clinical research, making trials more inclusive, efficient, and patient-centered.Real-Time Data Collection & Its Impact
One of the biggest advantages of wearables in clinical trials is the ability to collect real-time data. Instead of waiting for periodic check-ins or relying on patient memory, researchers can now access live, continuous data streams. This kind of data reduces the chances of recall bias, where patients may forget or misreport symptoms. It also helps detect subtle changes that might be missed during routine visits. Real-time data provides a clearer picture of how a patient is responding to treatment over time. Moreover, this continuous flow of data creates new opportunities for analysis. With the help of artificial intelligence and machine learning, researchers can uncover patterns and trends that were previously hidden. This leads to faster insights, smarter trial design, and more accurate results. For example, in a study on depression, wearable data might reveal that a patient’s physical activity and sleep patterns change several days before they report feeling worse. This early signal could lead to earlier intervention and better outcomes. The ability to gather and analyze real-time data is not just improving trials—it’s paving the way for a more responsive and adaptive healthcare system.Enhancing Patient Engagement & Compliance
Patient engagement has always been a challenge in clinical trials. Many participants drop out due to the burden of travel, complicated protocols, or lack of communication. Wearables help solve some of these problems by making participation easier and more interactive. For instance, wearable devices can send reminders for medication or prompt participants to answer short surveys. Some devices even offer gamified elements, rewarding users for reaching certain health goals. These small features can boost motivation and keep patients involved throughout the trial. Another key trend is BYOD, or Bring Your Own Device. This model allows participants to use their own smartphones or wearables instead of receiving new devices from the study team. BYOD reduces costs, simplifies logistics, and often leads to better compliance since users are already familiar with their devices. By integrating wearables into everyday life, trials become less disruptive. Participants feel more in control, and the study process becomes more flexible and convenient. Higher engagement leads to better data quality and more successful trials. Patient-centric design is no longer optional—it’s a must. And wearables are helping to make that a reality.
Decentralized Trials Powered by Wearables
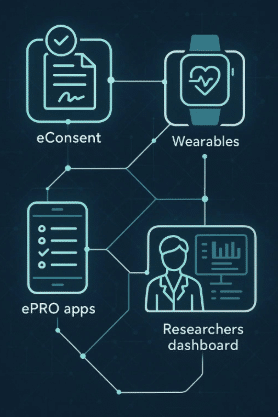
Decentralized clinical trials (DCTs) are trials that take place outside of traditional research sites. Instead of requiring patients to travel to a hospital or clinic, DCTs bring the trial to the patient through digital tools, home visits, and wearable technology. Wearables are a cornerstone of this model. They allow researchers to track patient health continuously, regardless of location. Combined with telemedicine, electronic consent, and mobile apps, wearables help create a seamless virtual trial experience. One of the biggest benefits of decentralized trials is access. Patients who live far from major research centers, or who can’t take time off work or caregiving duties, can now participate from home. This broadens the reach of clinical trials and leads to more diverse and representative data. Companies like Delve Health are leading the way in making decentralized trials a reality. Their solutions support wearable integration, remote data collection, and virtual patient engagement—all key elements of a modern, efficient trial. As the industry moves toward more flexible and patient-friendly models, decentralized trials supported by wearables are set to become the norm.Challenges and Considerations
While wearables bring many advantages to clinical trials, there are also challenges to consider. One major concern is data privacy. With so much personal health data being collected, it’s essential to follow strict regulations like HIPAA in the United States and GDPR in Europe. Sponsors must ensure that data is encrypted, anonymized, and securely stored. Another challenge is device compatibility. Different wearables may use different formats, sensors, and data outputs. Standardizing this information so it can be used across platforms and analyzed consistently is a complex task. Accessibility is also a factor. Not all participants are comfortable using digital devices, especially older adults or those with limited tech literacy. Studies must be designed with user-friendly interfaces and provide support to ensure all patients can participate fully. Finally, researchers must validate the accuracy of wearable data. While many devices are FDA-cleared, not all are. Choosing reliable, clinically validated devices is key to maintaining trial integrity. These hurdles are real, but they are not insurmountable. By partnering with experienced digital health providers and putting patients at the center of trial design, sponsors can overcome these challenges and unlock the full potential of wearable technology.Future Outlook: The Next 5 Years
The next five years are expected to bring even greater integration of wearables in clinical research. We’ll see more intelligent devices capable of measuring new biomarkers and offering deeper insights into health. AI and machine learning will play a bigger role in analyzing wearable data, identifying trends, and predicting outcomes. This could lead to more personalized trials, where protocols adapt to the individual rather than using a one-size-fits-all model. We can also expect more hybrid trials, combining in-person visits with remote monitoring. This flexibility will make trials faster, more cost-effective, and more aligned with patients’ lives. As more regulatory bodies embrace digital tools and as real-world evidence becomes a greater priority, wearables will no longer be a “nice to have” but a “must have” in clinical trials.Conclusion
Wearables are no longer just consumer gadgets. In the world of clinical research, they are powerful tools that are improving how we collect data, monitor health, and engage patients. They bring trials closer to everyday life, making studies more inclusive, accurate, and patient-friendly. As researchers and sponsors look for ways to make trials more efficient and effective, wearable technology offers a clear path forward. Whether it’s through digital biomarkers, remote monitoring, or decentralized trial models, the future of clinical trials is being shaped by these small but mighty devices. If you’re ready to explore how wearable technology can improve your research, consider working with a partner that specializes in virtual and decentralized trials. At Delve Health, we provide the tools and support to help you integrate wearables into your studies—making your research smarter, faster, and more patient-centric.Frequently Asked Questions
1. What are the benefits of using wearables in clinical trials?
Wearables offer continuous, real-time data collection, reducing reliance on clinic visits. They enhance patient engagement by integrating into daily life, improving compliance. Trials become more inclusive, as remote participation allows access for diverse populations. Overall, wearables streamline operations and improve the quality and accuracy of clinical data.
2. How do digital biomarkers improve healthcare outcomes?
Digital biomarkers, collected through wearables, provide high-frequency and objective insights into a patient’s health. They enable earlier detection of changes, support faster decision-making, and facilitate more personalized treatment. Their continuous nature also helps monitor long-term trends, enhancing both preventative care and the effectiveness of ongoing clinical interventions.
3. Is remote patient monitoring reliable for clinical studies?
Yes, remote patient monitoring is highly reliable when using validated devices and standardized protocols. It ensures accurate, real-time data capture and allows for early identification of issues. RPM enhances patient safety, supports faster responses to adverse events, and reduces the burden of frequent travel for study participants.
4. What is BYOD in clinical research and how does it work?
BYOD, or Bring Your Own Device, lets participants use their personal smartphones or wearables in clinical trials. This approach reduces equipment costs and training needs, increases convenience, and often boosts compliance. It simplifies data collection while maintaining participant comfort and familiarity with the technology being used.
5. How can I start incorporating wearables into my clinical trials?
To start using wearables, partner with a digital research platform like Delve Health. They assist with selecting the right devices, integrating data streams, ensuring regulatory compliance, and providing participant support. This helps you launch wearables-enabled trials smoothly, with confidence in the quality and scalability of your research design.