Clinical Trials come in multiple flavors, including virtual, hybrid, and fully site-based trials. The impact of COVID-19 on the industry of clinical research and drug delivery has interrupted nearly 80% of the studies, which challenged the ability to conduct trials safely.
This has led the industry to think of innovative ways to conduct clinical trials, including the ability for patients to conduct a clinical trial from the comfort of their home. Decentralized Trials has gained enormous momentum to help keep clinical trials moving forward, but what does that mean?
Prior to COVID-19, most clinical trials happened at a doctor’s office, including the need for patients to travel long distances from their home to the study site. Nearly 70% of patients enrolled in a clinical trial live at least 2 hours away from the nearest clinical site that is conducting the study, which is also a factor to why nearly 30% of patients drop out early.
How can a fully decentralized or hybrid trial approach help?
Improving Patient Diversity
Moving the needle from focusing only on patients within a specific hospital system to utilizing digital avenues and social media to engage a broader audience of patients. This means advertising to patients, caregivers and their doctors to peak their interest of a new novel drug that can be beneficial for them and other patients.
In a study conducted by American Society of Clinical Oncology, where they were able to recruit 1178 patients in 3 months using digital media technologies https://ascopubs.org/doi/abs/10.1200/JCO.2016.34.18_suppl.LBA1519
Remote Consent
Allow for remote consent in a way that is adequate to exchange information, using mobile solutions, video consent, and a way to allow the patient or guardian to electronically sign the informed consent
Remote or In-Home Visits
Depending on the type of clinical trial and the nature of the visit, many visits can be conducted virtually by using a video visit and utilizing in-home nurses to collect blood samples. This can mean shipping mobile solutions and wearables to collect the appropriate data needed for the study. Utilizing our network of nurses (nearly 1 millions nurses throughout the US), we are able to help conduct trials faster and keep your study moving forward.
Utilize Wearables
Remotely monitor patients and collect digital endpoint data automatically, including heart rate, motion, sleep, activity and step counts. Given that patients are away from the doctor’s office 99% of the time, the use of wearables and sensors allows the investigators to remotely monitor their patients more accurately.
Remote patient monitoring may have potential to improve patient safety by generating early warnings for deterioration to study team.
Since COVID-19, the use of wearables and mobile technologies in clinical trials has increased by 22%.
Mobile Apps and SMS
The average person using a smart phone touches their phone over 2700 times a day. Using text messages has been a powerful utility to engage with patients and study teams to provide real-time notification and updates to patients and study teams in regards to possible adverse event based on abnormalities in vital signs.
In September 2020, Modern Healthcare published some highlights from research conducted by SR Health by Solutionreach, indicating that patients have made a significant shift in their communication preferences during the COVID-19 outbreak. Interest in receiving phone calls from healthcare providers dropped by about 14 percent. At the same time, patients are expressing much more interest in text messaging as an alternative to phone calls. Texts are convenient and offer greater flexibility in timing for participants in clinical trials.
Interested to learn more about conducting a fully decentralized or hybrid clinical trial? Reach out to the experts at Delve Health (www.delvehealth.com)
About the author
Wessam Sonbol is a CEO at Delve Health. At Delve Health, we focus on bringing trials closer to patients, using wearables, mobile solutions and patient adherence programs. We develop innovative technologies to remotely capture data from patients, consent them and follow them from study inception to completion.
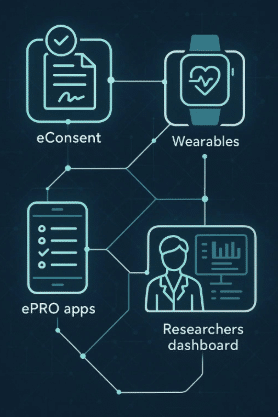
Our Clinical StudyPal is a decentralized platform that can be utilized with a mix of modules for your clinical trial, including randomization, manage study drug supply (RTSM), electronic consent, patient-reported outcomes (ePRO), real-time notification, adherence analytics, and virtual video visits.
Clinical StudyPal can virtualize many aspects of your trial, from providing remote consent using video, capture your primary and secondary endpoint data via a mobile device, score the outcomes measure, and provide real-time notification to your study team of any adverse events captured.