SERVICES
WEARABLE SENSORS
WHAT ARE WEARABLES IN CLINICAL TRIALS?
Wearable sensors in clinical trials and digital health can provide patient monitoring, surveillance, screening, diagnosis and assistance with treatment, post-treatment and on-going management. These devices also determine, and confirm, efficiency of treatment based on real-time physiological feedback.

Improving Clinical Trials Through WEARABLE SENSORS
The widespread adoption of wearable sensing technology, both in our personal and professional lives, means the healthcare and clinical research industries can benefit from a product that is already “mainstream”—increasing the use of artificial intelligence (AI) in software solutions for wearables and remote biosensors.
This has led to clinical sites’ investigation of and subsequent implementation of wearable technology in clinical trials. Currently, about 10% to 15% of trials are incorporating wearable devices, primarily to collect data as exploratory endpoints (PharmaVoice); and, according to research by Berg Insights, the number of remotely monitored patients is expected to reach 50.2 million by this year.
EXPLORE THE ENDLESS POSSIBILITIES OF OUR WEARABLE SENSORS
Unparalleled Data
Collection
Our innovative, device-agnostic, end-to-end platform seamlessly integrates virtually any wearable or connectable device and/or biosensor into patients’ daily lives, passively capturing a wealth of valuable data with minimal disruption. From heart rate (HR) and sleep patterns to activity levels and vital signs, our wearable vital sign monitor leverage many FDA approved devices to provide a comprehensive picture of patients’ health—enabling researchers and healthcare providers to make informed decisions and drive meaningful outcomes.
Patient-Centric
Approach
Our technology platform also provides diverse and underrepresented patient populations access to quality healthcare and clinical trials—closing the inequality gap created by social determinants of health (SDOH)—removing barriers and increasing access to all patients worldwide.
Raw Data Capture (RDC)
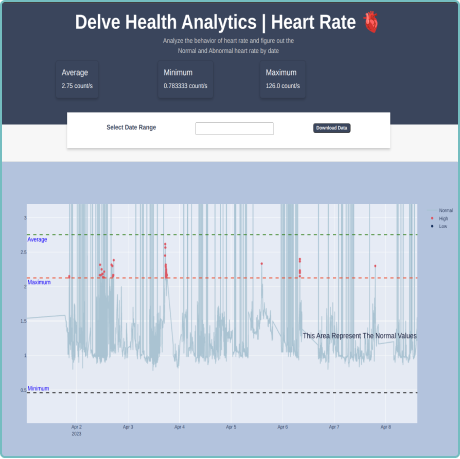
We understand the importance of raw data in clinical trials. Our configurable platform captures and stores raw data, providing researchers with a wealth of detailed information for in-depth analysis. This raw data capture allows for a comprehensive understanding of patients’ physiological parameters and behaviors, enabling researchers to utilize and develop their own algorithms against the raw data collected. This benefit supports researchers’ efforts and provides them with the opportunity to uncover hidden patterns and correlations. With access to unfiltered data, you can extract valuable insights, drive scientific breakthroughs and make data-driven decisions with confidence. By embracing raw data capture, Delve Health empowers you to explore the full potential of clinical trials wearable devices, all from within a powerful, single analytical application—driving ease of use, better full data sets, quicker results, better outcomes and ultimately safely speeding-up the timelines to offering medications and therapies in the marketplace for patients.
Real-Time Monitoring
With real-world data (RWD) in real-time streaming, wearable remote patient monitoring empowers researchers to monitor patients’ progress remotely. This eliminates the need for frequent site visits and enables prompt interventions when necessary. By closely tracking patients’ health parameters, we optimize trial outcomes and enhance patient safety.

Seamless Integration
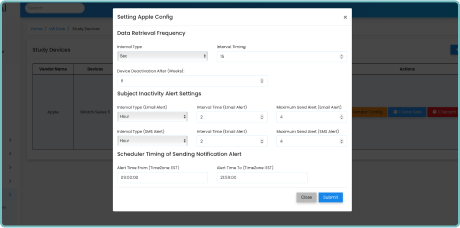
Integrating with existing clinical trial platforms is effortless with Delve Health’s solution. Our remote patient monitoring wearables seamlessly synchronize with your preferred data management systems, ensuring streamlined workflow. We provide secure, encrypted data transmission, protecting patient privacy, as we are HIPPA, GDPR, and CFRpart11 compliant, while facilitating efficient data analysis.
Empowering Research Insights
Our comprehensive analytics platform empowers researchers with actionable insights derived from wearable sensor data. Unlock the potential of big data and harness it to uncover patterns, correlations and predictive markers. By combining clinical expertise with advanced analytics, we help you make groundbreaking discoveries and push the boundaries of medical knowledge.
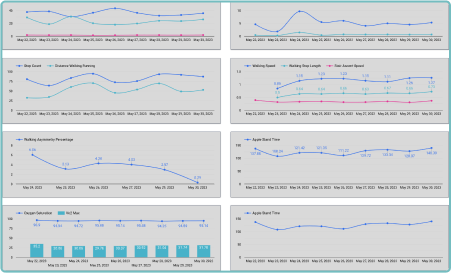
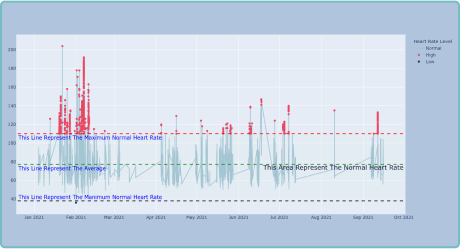
Enhanced Trial Efficiency
By leveraging wearable devices and biosensors in clinical trials, Delve Health significantly improves trial efficiency. Our innovative technology reduces data collection time, minimizes errors and accelerates the analysis process. Researchers gain deeper insights faster, enabling quicker decision-making and facilitating the development of life-changing therapies.
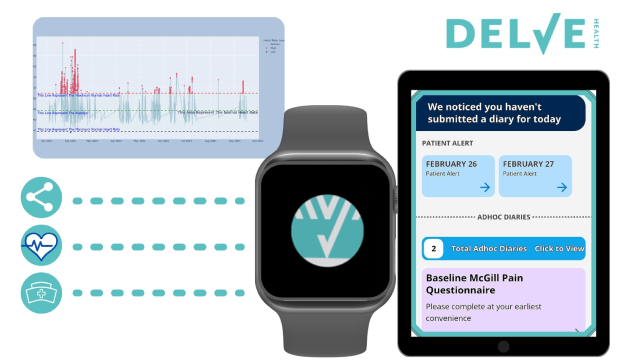
Are You Looking to Transform the Way Clinical Research is Conducted?
Delve Health’s hybrid model of BYOD and fully-configured, agnostic devices has enabled patients, clinicians and caregivers to report outcomes for more granular endpoint data—utilizing more sensitive measures than traditional clinical scales; our wearables program makes it easier for your team to conduct extended remote studies and easier for patients to engage.
Frequently Asked Questions
Wearable devices used in clinical trials include smartwatches, fitness trackers, biosensors, and wearable ECG monitors. These devices help in monitoring various physiological parameters and collecting real-time data.
Wearable technology enhances clinical trials by collecting data as exploratory endpoints, increasing patient engagement, and enabling remote monitoring. This leads to more comprehensive data collection, reduced site visits, and optimized trial outcomes.
Wearable devices can collect various types of data, including heart rate, sleep patterns, activity levels, and vital signs. This data provides a comprehensive picture of patients’ health, enabling informed decision-making by researchers and healthcare providers.
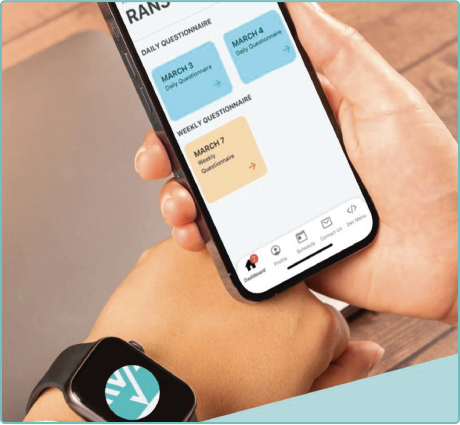
Delve Health’s platform, Clinical StudyPal, is designed to be intuitive, engaging, non-intrusive, and easy to use. This reduces patient burden, ensures higher compliance rates, and generates more reliable, actionable data.
Raw data capture provides researchers with detailed information for in-depth analysis, supporting efforts to uncover hidden patterns and correlations. This allows for scientific breakthroughs and data-driven decisions with confidence.




























