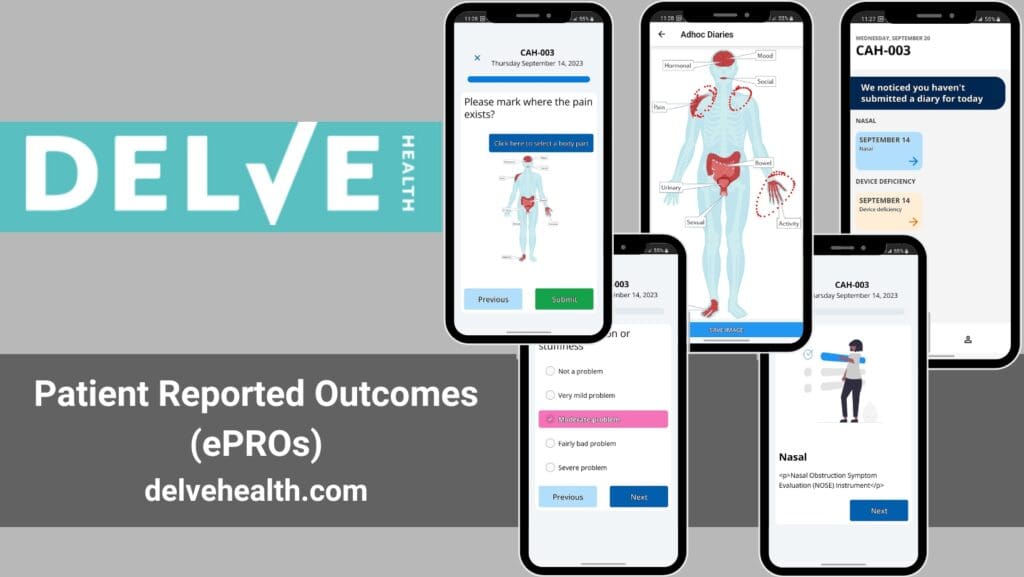
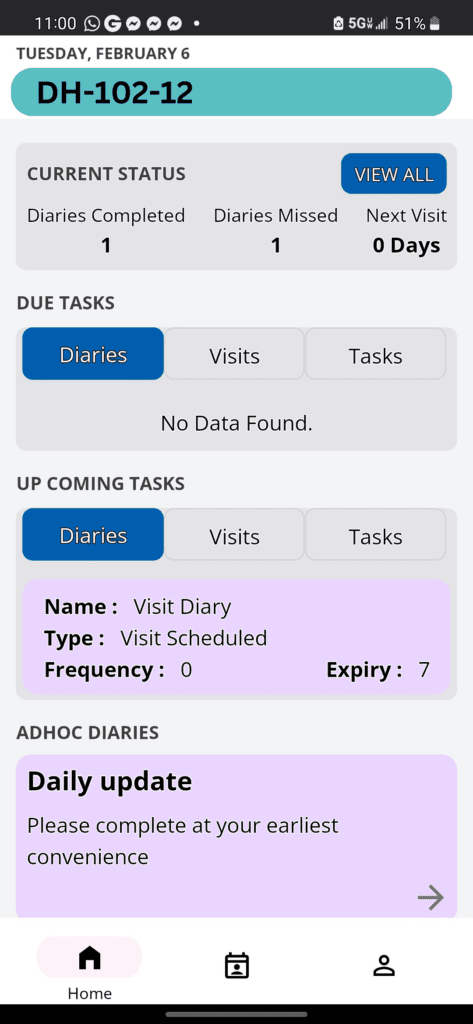
Delve Health’s Deployment of Patient Reported Outcomes in Oncology: Pathways to Success and Enhanced Patient Compliance
Introduction In the intricate field of oncology, understanding patient perspectives—how they feel and function—is crucial. Delve Health stands at the forefront of integrating Patient Reported Outcomes (PROs) into clinical trials, aiming to elevate patient care and enhance treatment efficacy. This approach not only aligns with modern healthcare’s patient-centered model but also boosts compliance and overall […]