What is eCOA and ePRO?
eCOA (Electronic Clinical Outcome Assessment) and ePRO (Electronic Patient-Reported Outcome) are crucial components of modern clinical trials.
eCOA: Capturing Comprehensive Clinical Trial Data Digitally
eCOA is a digital approach to capturing a wide range of clinical trial data. It encompasses various types of assessments including:
- Patient-reported outcomes (PROs)
- Clinician-reported outcomes (ClinROs)
- Observer-reported outcomes (ObsROs)
- Performance outcomes (PerfOs)
This comprehensive method ensures that all necessary data types are efficiently captured and analyzed.
ePRO: Putting Patients at the Center of Data Collection
On the other hand, ePRO is a subset of eCOA. It specifically focuses on collecting data directly from patients using electronic devices such as smartphones, tablets, or web-based platforms. This method puts patients at the center of the data collection process, ensuring their voices are heard accurately and timely.
Importance of Electronic Data Capture in Clinical Trials
Electronic data capture plays a crucial role in clinical trials by:
- Enhancing data quality and accuracy
- Reducing the risk of data entry errors
- Streamlining data collection and analysis processes
- Improving patient compliance and engagement
By leveraging tools like eCOA and ePRO, researchers can ensure more reliable and actionable insights from their studies.
To facilitate patient engagement effectively in clinical trials, some organizations offer specialized services like Delve Health’s Patient Concierge Services. These services provide a dedicated point of contact to help patients navigate the complexities of clinical trials while providing essential support throughout their participation.
Understanding eCOA
Electronic Clinical Outcome Assessment (eCOA) is a comprehensive digital tool designed to capture a variety of data types in clinical trials. The primary data types captured by eCOA include:
Patient-Reported Outcomes (PROs)
These are direct reports from patients about their health condition, without interpretation by clinicians or anyone else. Examples:
- Symptom diaries are where patients log their daily experiences with pain or discomfort.
- Quality of Life (QoL) questionnaires measuring overall well-being.
Clinician-Reported Outcomes (ClinROs)
These are assessments made by healthcare professionals based on their observations and interactions with the patient. Examples:
- Clinical examination results, such as doctor’s notes on physical health status.
- Medical imaging interpretations, like X-rays, are analyzed by radiologists.
Observer-Reported Outcomes (ObsROs)
Reports are provided by someone other than the patient or clinician, often a caregiver or family member. These are particularly useful for capturing data in populations unable to self-report, such as young children or individuals with cognitive impairments. Examples:
- Parent-reported symptom checklists for pediatric studies.
- Caregiver observations regarding the daily functioning of dementia patients.
Performance Outcomes (PerfOs)
These involve tasks performed by the patient according to instructions provided by the clinician, which are then evaluated to measure specific outcomes. Examples:
- Timed walk tests to assess mobility.
- Cognitive function tests, such as memory recall exercises.
Understanding these different data types is crucial because each provides unique insights into the patient’s experience and the effectiveness of the treatments being studied.
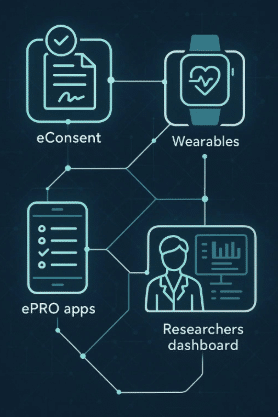
Methods of Data Collection in eCOA
eCOA employs various methods to gather this diverse range of data, leveraging technology to enhance accuracy and efficiency. Common methods include:
1. Electronic Diaries and Questionnaires
Patients can use smartphones, tablets, or web-based platforms to complete diaries and questionnaires at home or on the go. This method ensures real-time data capture and reduces recall bias.
2. Interactive Voice Response Systems (IVRS)
This telephone-based method allows patients to report their symptoms or health status via automated calls, making it accessible for those who may not be comfortable using digital devices.
3. Wearable Devices
Fitness trackers and other wearable technology can continuously monitor physiological parameters such as heart rate, activity levels, and sleep patterns, providing objective data that complements self-reported information.
4. Electronic Clinician Assessments
Clinicians enter observations and assessments directly into electronic systems during patient visits, ensuring immediate availability of data for analysis.
Using multiple methods of data collection enriches the dataset, providing a more comprehensive picture of patient outcomes. For instance:
- In an eCOA study for a new arthritis medication, clinicians might perform joint flexibility tests during visits (ClinROs) while patients log their pain levels daily (PROs) using a mobile app.
- A study on Alzheimer’s disease could involve caregiver reports on behavioral changes (ObsROs) alongside performance-based cognitive tasks (PerfOs) conducted through an electronic interface at regular intervals.
Each method offers distinct advantages and when combined within eCOA frameworks, they enhance the robustness and reliability of clinical trial outcomes.
By integrating these diverse data types and collection methods, eCOA stands out as an essential tool in modern clinical trials. It not only improves data quality but also aligns closely with regulatory requirements for patient-centric evidence.
Methods of Data Collection in eCOA
eCOA (Electronic Clinical Outcome Assessment) uses various methods to collect comprehensive data in clinical trials. These methods ensure diverse and robust insights, capturing different aspects of patient health and treatment outcomes.
Data Collection Methods
1. Clinician-Reported Outcomes (ClinROs)
- Collected through direct input from healthcare professionals.
- Examples include:
- Clinical assessments are performed during patient visits.
- Observational notes on patient behavior and condition.
2. Patient-Reported Outcomes (PROs)
- Direct feedback from patients regarding their health status, symptoms, or quality of life.
- Examples include:
- Surveys or questionnaires are administered via smartphones or tablets.
- Daily diaries where patients record their experiences.
3. Observer-Reported Outcomes (ObsROs)
- Data is provided by someone other than the patient or clinician, such as a caregiver.
- Examples include:
- Reports on a patient’s daily activities and physical capabilities.
4. Performance Outcomes (PerfOs)
- Objective measures of a patient’s performance in specific tasks.
- Examples include:
- Timed walk tests to assess mobility.
- Cognitive function tests are conducted through interactive software.
Importance of Multiple Data Collection Methods
Using a combination of these methods ensures:
- Comprehensive Data Capture: Different perspectives enrich the dataset, providing a holistic view of patient health.
- Increased Reliability: Cross-validation of data from various sources enhances accuracy and consistency.
Examples in eCOA Studies
- Clinician Assessments:
A clinician may use an electronic tablet to record observations during a routine check-up. This real-time data entry helps reduce errors compared to traditional paper-based methods.
- Performance Tests:
Patients might be asked to perform specific physical tasks, such as walking a certain distance, while their performance is recorded electronically. These objective measures provide valuable insights into treatment efficacy.
Integrating multiple data collection methods in eCOA not only streamlines the process but also leads to more reliable and actionable insights.
Understanding ePRO
ePRO, or Electronic Patient-Reported Outcome, is a crucial component in modern clinical trials. It specifically focuses on capturing patient-reported data directly from patients through electronic devices like smartphones, tablets, or web-based platforms. This digital approach to data collection ensures that the patient’s voice is heard more accurately and promptly.
Definition and Purpose of ePRO
ePRO refers to the use of electronic systems to collect data directly from patients about their health condition, symptoms, quality of life, and treatment satisfaction. The primary purpose of ePRO is to:
- Enhance the accuracy and timeliness of patient-reported data
- Minimize errors associated with traditional paper-based reporting
- Provide real-time access to patient feedback for researchers and clinicians
Role of ePRO in Capturing Patient-Centric Data in Clinical Trials
Patient-centric data is vital for evaluating the efficacy and safety of medical treatments. ePRO plays a significant role in this process by:
- Allowing patients to report their experiences and symptoms conveniently
- Enabling continuous monitoring of patient health outside clinical settings
- Facilitating more personalized treatment plans based on real-time data
Advantages of Using ePRO
Switching to ePRO offers several benefits over conventional methods. Here are some key advantages:
Convenience for Patients
Patients can report their outcomes using familiar devices such as smartphones and tablets, making the process less cumbersome and more consistent.
Real-Time Data Collection
Electronic devices allow for instant data capture, reducing the lag time between patient experiences and clinician review.
Enhanced Data Accuracy and Reliability
Digital entry minimizes transcription errors that often occur with paper-based methods. Automated checks can ensure that all required fields are completed correctly.
Improved Compliance
Automated reminders via electronic devices can encourage patients to complete their reports on time, leading to higher compliance rates.
Streamlined Data Management
Data collected electronically can be easily integrated into centralized databases, facilitating efficient analysis and reporting.
Benefits of Collecting Patient-Reported Data Through Electronic Devices
Using electronic devices for patient-reported data collection has transformative impacts on clinical research:
Increased Engagement
Patients are more likely to engage with user-friendly interfaces on their personal devices compared to filling out lengthy paper questionnaires.
Accessibility
Electronic platforms can be designed to accommodate different languages and accessibility needs, ensuring broader participation.
Security
Digital systems offer advanced encryption and security measures to protect sensitive patient information.
Increased Accuracy, Reliability, and Compliance with ePRO
The shift towards ePRO not only enhances the quality of the data collected but also boosts overall study reliability:
Precision in Reporting
Patients can provide more accurate reports when they use electronic tools that guide them through each question systematically.
Consistency Over Time
Regular prompts help maintain consistency in patient responses throughout the trial period.
Robust Data Integrity
Automated validation checks reduce inconsistencies and missing data entries, ensuring a robust dataset for analysis.
Embracing ePRO transforms how patient-reported outcomes are captured, offering a blend of convenience, precision, and efficiency that traditional methods struggle to match. This makes it an indispensable tool for achieving reliable and high-quality clinical trial results.
Key Differences Between eCOA and ePRO
Understanding the key differences between eCOA and ePRO helps in selecting the right tools for clinical trials.
Scope Difference
eCOA
Encompasses a broad range of data types, including:
- Patient-Reported Outcomes (PROs): Data provided directly by patients.
- Clinician-Reported Outcomes (ClinROs): Assessments made by healthcare professionals.
- Observer-Reported Outcomes (ObsROs): Observations made by someone other than the patient or clinician, often a caregiver.
- Performance Outcomes (PerfOs): Results from objective tests measuring a patient’s performance.
ePRO
Specifically focuses on data reported directly by patients. This makes it a subset of eCOA, concentrating solely on:
- Patient-Reported Outcomes (PROs).
Comparison of Data Types
eCOA
Collects diverse data types that offer a holistic view of the patient’s condition from multiple perspectives:
- ClinROs provide professional insights from clinicians.
- ObsROs capture observations from caregivers or others close to the patient.
- PerfOs measure objective performance metrics, contributing to comprehensive data collection.
ePRO
Focuses exclusively on patient-centric data through electronic means:
- Enhances accuracy and reliability since patients report their symptoms, experiences, and quality of life directly.
- Increases compliance due to the convenience of using smartphones, tablets, or web platforms.
Utilizing both eCOA and ePRO ensures robust data capture by leveraging diverse sources and perspectives.
Importance of eCOA and ePRO in Ensuring Data Quality
Significance in Clinical Trials
1. Enhanced Data Quality
Utilizing eCOA and ePRO ensures that clinical trial data is captured with high precision. Electronic data capture minimizes errors linked to manual entry, leading to more reliable datasets.
2. Improved Accuracy and Integrity
Advanced capture techniques like real-time data logging improve the integrity of the information collected. This is crucial for making informed decisions based on accurate and current data.
Reduction of Risks
1. Minimizing Paper-Based Errors
Traditional methods often involve paper forms that are prone to misentries, loss, and damage. Transitioning to electronic systems reduces these risks substantially.
2. Automated Processes
Automated checks and prompts in electronic systems help ensure that no critical data points are missed, reducing human error.
Streamlined Data Collection and Analysis
1. Efficient Management
Using electronic systems simplifies the management of large volumes of data. Centralized databases make it easier to organize, retrieve, and analyze information quickly.
2. Real-Time Insights
Instant access to up-to-date information allows for timely insights and quicker decision-making processes. This can be particularly beneficial in adaptive trial designs where ongoing modifications are necessary.
Leveraging eCOA and ePRO methodologies not only enhances the quality of clinical trial data but also streamlines the entire process from collection to analysis. Embracing these advanced tools reduces risks associated with traditional methods, paving the way for more efficient and accurate clinical research.
Conclusion
Combining eCOA and ePRO can completely transform clinical research. By using these technologies, researchers can collect comprehensive, high-quality data while prioritizing the needs of the patients.
Key Takeaways:
- Enhance Data Quality: Make use of both eCOA and ePRO to gather diverse, accurate, and reliable data.
- Improve Compliance: Electronic methods of capturing data help in increasing participant adherence and engagement.
- Optimize Processes: Make your clinical trials more efficient with advanced techniques for collecting and manaing data.
To learn more about how Delve Health can support your research needs with state-of-the-art technology designed to improve every aspect of your study, check out our offerings on electronic data capture.