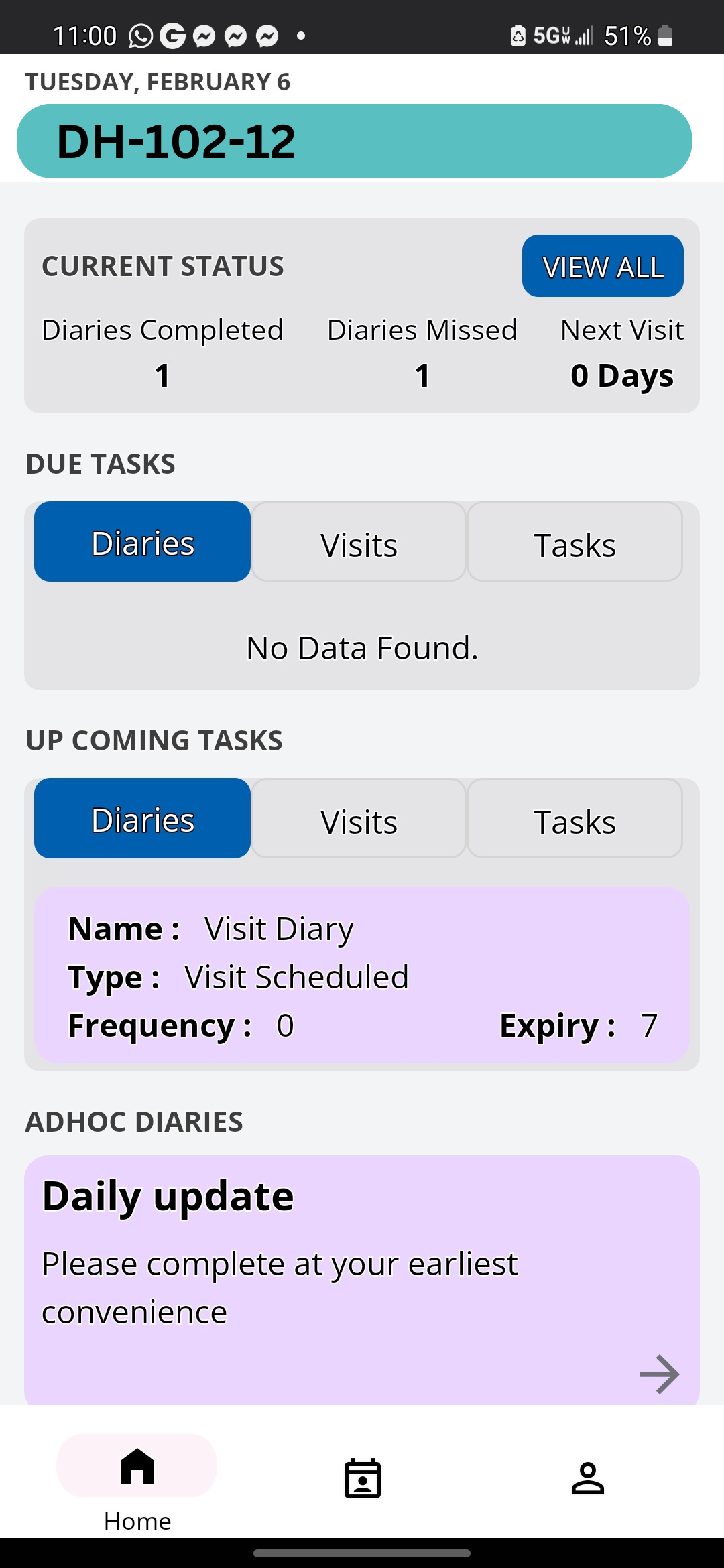
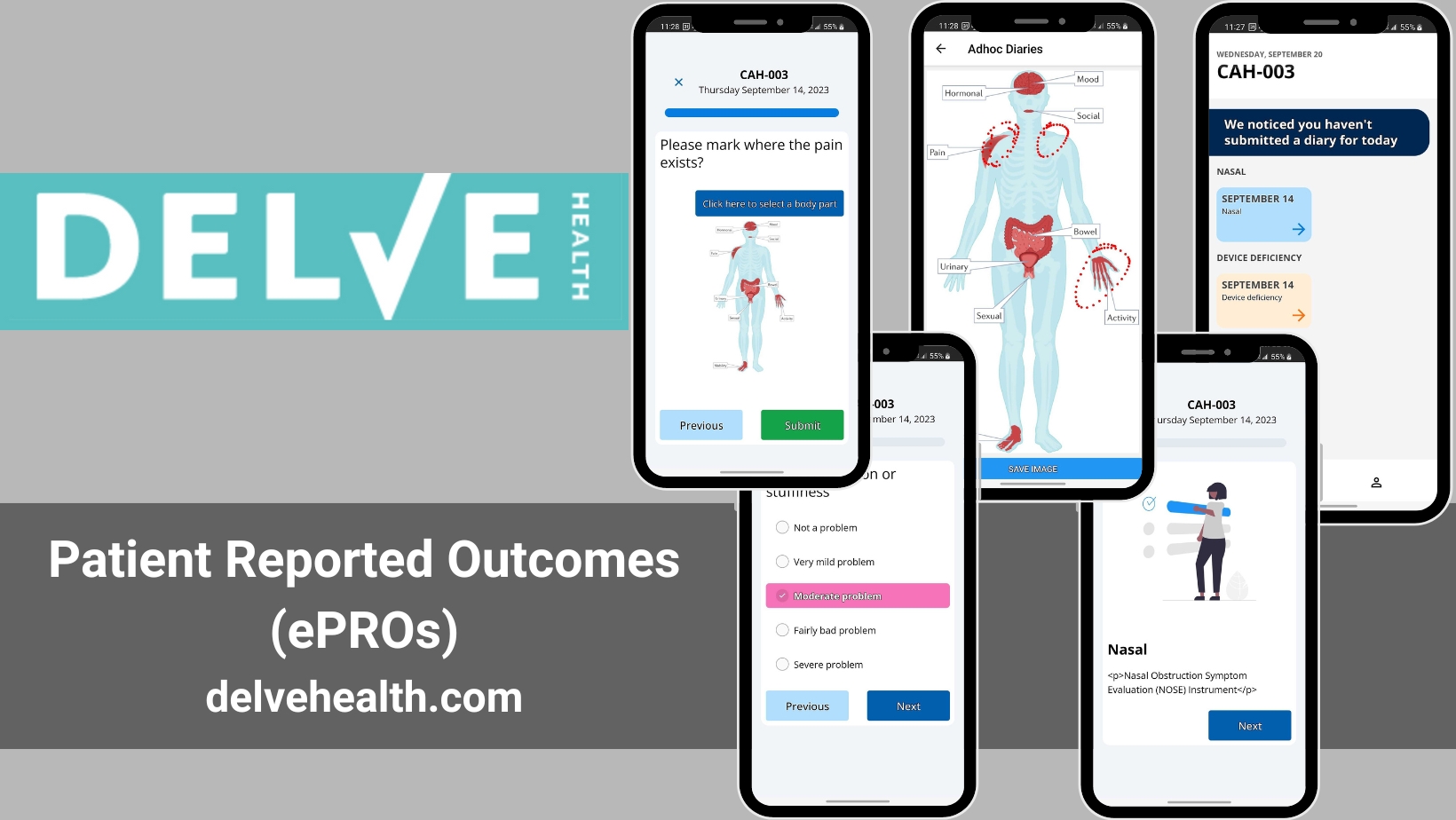
With our fully configurable ePRO/eCOA solution, we help assure your team gets the highest quality patient-reported outcomes for your study. The movement from paper-based to ePRO data capture has enhanced the integrity and accuracy of clinical trial data and is encouraged by regulators. We offer flexible, automated notifications and robust patient-reported outcome tools that give you more accurate and complete data. Here are some reasons why our clients utilize our platform, and solutions.
Clinical StudyPal meets all global regulations for collecting clinical trial data including ICH/GCP, 21 CFR, GDPR, and HIPAA. More about our capabilities – we can utilize home clinical visits (using a network of 1million nurses) and mobile application technology to screen and gain consent from patients using video-conferencing, and allowing nurses to collect data through an app and supply patients with ePROs Get your demo today. Click here Calendar: https://calendly.com/delvehlth/30min |
Related articles


Delve Health’s Wearable-as-a-Service: Revolutionize Your Clinical Trials
Enabling wearable as a service for clinical trials to reduce costs and improve real world evidence creation.

Delve Health’s Deployment of Patient Reported Outcomes in Oncology: Pathways to Success and Enhanced Patient Compliance


Case Study: Leveraging Delve Health Platform for Parkinson’s Study with Wearables Monitoring Dyskinesia


Digital Endpoints and Patient Outcomes in Clinical Trials: Understanding the Differences and Similarities


The Importance of Patient Engagement in Clinical Trials and Innovative Strategies for Enhancement