The world of clinical research is experiencing a significant change. Decentralized Clinical Trials (DCTs) are changing the way we conduct medical research by shifting traditional site-based activities to participants’ homes and local healthcare facilities.
Instead of making frequent trips to research centers, participants can now contribute to important medical research from the comfort of their own homes. DCTs use digital technologies to prioritize the needs of patients, eliminating geographical barriers and increasing access to clinical studies.
This change is not only convenient but also improves the quality of data collected during these trials. For example, Delve Health’s platform has demonstrated how digital innovation and patient involvement can greatly enhance data quality and study results in clinical trials.
At the core of this transformation is patient engagement – a crucial element that determines the success of a clinical trial. When participants are actively involved, they are more likely to complete the trial, follow study protocols accurately, report data reliably, and contribute meaningfully to research outcomes.
Enter wearable technology – the innovative tools that connect researchers and participants. From smartwatches to biosensors, these devices silently monitor health metrics, gathering valuable information while keeping participants engaged in their own well-being.
Think of wearables as personal health assistants that:
- Track vital signs continuously
- Measure physical activity levels
- Offer instant feedback on health behaviors
- Facilitate direct communication with research teams
This digital transformation in clinical trials goes beyond just data collection – it redefines the entire experience for participants, making it more inclusive, interactive, and impactful for everyone involved. Another significant advancement is the integration of automation in these processes. Automated workflows in digital healthcare and connected devices have streamlined operations further, ensuring smooth data collection and patient monitoring.
As we observe International Clinical Trials Day 2023, it’s important to recognize the value of these trials in advancing medical innovation. With these developments on the horizon, the future of clinical research looks bright – leading us towards more effective and patient-centered studies.
Understanding Decentralized Clinical Trials (DCTs)
Decentralized Clinical Trials represent a revolutionary shift in medical research methodology. These innovative studies allow participants to engage in clinical trials from their homes or local healthcare facilities, eliminating the need for frequent visits to traditional research centers.
Key Components of DCTs:
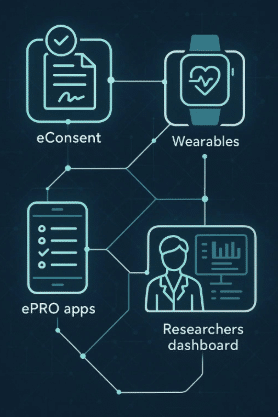
- Remote data collection through digital health technologies, such as using devices like the Apple Watch for real-time health monitoring
- Virtual study visits and telehealth consultations
- Direct-to-patient drug delivery
- Local healthcare provider participation
- Digital consent processes, which are an integral part of the eConsent in virtual clinical trials
Traditional clinical trials often require participants to travel long distances to research sites, creating significant barriers to participation. DCTs break down these geographical limitations by bringing the trial to the participant.
Patient-Centric Advantages:
- Reduced travel burden
- Flexible scheduling options
- Familiar environment for participation
- Greater access for diverse populations
- Simplified participation process
The accessibility of DCTs opens doors for previously underrepresented groups in clinical research. Rural communities, mobility-challenged individuals, and working professionals can now participate without disrupting their daily routines.

Traditional vs. DCT Approach:
| Aspect | Traditional Trials | Decentralized Trials |
|---|---|---|
| Visit Type | Site-based visits | Remote monitoring |
| Data Management | Paper documentation | Digital data capture |
| Geographical Reach | Limited geographical reach | Broader participant access |
| Scheduling | Rigid scheduling | Flexible participation |
| Monitoring | In-person monitoring | Real-time digital tracking |
DCTs leverage digital technologies to create a more efficient research environment. This modern approach reduces study timelines, decreases costs, and improves data quality through continuous monitoring and real-time collection.
The integration of local healthcare providers into DCTs creates a hybrid model that combines the benefits of traditional medical oversight with innovative digital solutions. This approach maintains high-quality care standards while maximizing participant convenience and engagement.
The Role of Technology in DCTs
Digital innovation has transformed clinical trials, introducing powerful tools that make research processes more efficient and improve participant experiences. Let’s take a look at the key technologies that are reshaping modern DCTs:
Health Apps: Your Digital Research Companion
Health apps are mobile applications designed to support clinical trials by providing participants with various features and functionalities. These apps serve as a digital companion for participants throughout the study, offering convenience and accessibility. Here are some key features of health apps:
- Real-time symptom tracking
- Medication adherence monitoring
- Digital diary entries
- Automated appointment reminders
- Direct messaging with research teams
Telehealth Platforms: Breaking Down Distance Barriers
Telehealth platforms play a crucial role in facilitating remote interactions between researchers and participants. These platforms leverage technology to overcome geographical limitations and enable effective communication. Here are some key features of telehealth platforms:
- Virtual consultations with research staff
- Remote health assessments
- Digital consent processes
- Online educational resources
- Video-based adverse event reporting
These technological solutions create a seamless research experience by:
- Reducing the need for in-person visits by up to 80%
- Enabling 24/7 data collection
- Providing instant access to study information
- Supporting multi-language capabilities
- Facilitating real-time data analysis
The integration of artificial intelligence and machine learning enhances these platforms by:
- Identifying patterns in participant behavior
- Predicting potential dropouts
- Suggesting personalized engagement strategies
- Automating routine communications
- Detecting data anomalies
Mobile health technologies have demonstrated remarkable success in DCTs:
- 62% increase in participant retention
- 45% reduction in study timeline
- 33% decrease in administrative burden
- 75% improvement in data quality
- 40% cost reduction compared to traditional methods
These digital tools work together to create an interconnected ecosystem that supports both researchers and participants throughout the trial journey. The technology stack continues to evolve, incorporating new features and capabilities to meet the growing demands of modern clinical research.
Wearables: A Key Component in Patient Engagement
Wearable technology has transformed how data is collected in decentralized clinical trials, creating a seamless connection between participants and researchers. However, dealing with wear compliance issues is still a challenge. Let’s take a closer look at the various types of wearables that are changing the landscape of clinical research:
Popular Wearable Devices in Clinical Trials:
- Smart Watches: Track heart rate, activity levels, sleep patterns
- Biosensors: Monitor vital signs, biochemical markers, physiological changes
- Smart Patches: Measure temperature, medication adherence, movement patterns
- Fitness Trackers: Record steps, exercise intensity, caloric expenditure
These devices provide participants with immediate access to their health metrics through user-friendly interfaces. A participant can track their heart rate variability, sleep quality, or physical activity levels with just a glance at their device.
Real-Time Health Insights
Wearables generate continuous streams of health data, offering:
- Instant feedback on physical responses to treatment
- Early detection of adverse reactions
- Personalized health trends and patterns
- Active engagement in the trial process
The continuous monitoring capabilities of wearables benefit both sides of the research equation:
For Participants:
- Greater awareness of their health status
- Immediate alerts for concerning changes
- Active involvement in their health journey
- Reduced need for in-person visits
For Researchers:
- Rich, objective data collection
- Rapid identification of treatment effects
- Higher quality, more consistent data points
- Better understanding of treatment impacts in real-world settings
The integration of AI and machine learning with wearable technology enables sophisticated pattern recognition and predictive analytics. These advanced features help identify subtle health changes that might go unnoticed in traditional trial settings.
Enhancing Communication Through Wearables
Wearable devices create dynamic communication channels between research participants and clinical trial teams. These devices act as digital bridges, enabling real-time interactions and immediate response capabilities.
Direct Participant-Researcher Connection
- Instant alerts notify researchers of significant health changes
- Built-in messaging systems allow quick question-and-answer exchanges
- Automated reminders help participants stay on track with trial protocols
- Virtual check-ins replace traditional site visits

Real-Time Data Sharing Benefits
- Participants gain immediate insights into their health metrics
- Researchers can spot trends and patterns as they emerge
- Quick intervention possibilities when abnormal readings occur
- Reduced need for retrospective data collection
The integration of feedback loops transforms passive data collection into active engagement:
Personalized Health Insights
- Daily activity summaries
- Progress tracking toward health goals
- Customized recommendations based on collected data
Interactive Features
- Two-way messaging capabilities
- Video consultation options
- Digital diary entries
- Symptom tracking tools
Building Trust Through Transparency
Wearables provide participants with unprecedented access to their own health data. This transparency builds trust and encourages active participation in the trial process. Participants can:
- Review their contributed data in real-time
- Track their compliance with study protocols
- Understand how their participation impacts research goals
- Feel more connected to the study’s progress
Smart algorithms analyze incoming data to generate personalized engagement strategies. These tailored approaches help maintain participant interest and commitment throughout the trial duration. The continuous flow of information creates a collaborative environment where participants feel valued as active contributors to medical research.
Benefits of Wearables in Decentralized Clinical Trials
The integration of wearables in decentralized clinical trials has transformed research outcomes through three key advantages: increased participation, cost reduction, and improved data quality.
Enhanced Participant Diversity and Engagement
Recent studies show a 60% increase in participant retention when wearables are incorporated into clinical trials. This significant boost stems from:
- Reduced travel requirements
- Simplified data collection processes
- Real-time health insights that keep participants invested
- Greater accessibility for diverse populations
Cost-Effective Research Solutions
The financial impact of wearable technology in clinical trials is substantial:
- 30-40% reduction in site monitoring costs
- 25% decrease in data collection expenses
- Minimized need for physical site visits
- Streamlined administrative processes
Superior Data Quality Through Real-Time Monitoring
Wearable devices have transformed data collection accuracy:
- 97% data completion rates compared to 71% with traditional methods
- Elimination of manual data entry errors
- Continuous monitoring captures previously missed health events
- Real-time data validation reduces post-trial cleaning requirements
Research Impact Metrics
Clinical trials utilizing wearables have demonstrated:
- 75% faster participant recruitment
- 49% increase in protocol adherence
- 3x more data points collected per participant
- 80% reduction in missing data
The implementation of wearable technology creates a win-win scenario for both researchers and participants. Research organizations benefit from streamlined operations and reduced costs, while participants enjoy a more convenient and engaging trial experience. These advantages contribute to higher quality research outcomes and accelerated study timelines.
Regulatory Considerations for Using Wearables
The integration of wearables in decentralized clinical trials demands strict adherence to regulatory frameworks.
FDA Regulations
The FDA’s 21 CFR Part 11 establishes essential requirements for electronic systems, including:
- Electronic signatures validation
- Audit trail maintenance
- System security protocols
- Data backup procedures
GDPR Compliance
For trials involving European participants, GDPR compliance adds another layer of requirements:
- Data minimization: Collecting only necessary health information
- Purpose limitation: Using data solely for specified trial objectives
- Storage restrictions: Implementing time-limited data retention policies
Validation Processes
Clinical trial sponsors must implement robust validation processes for wearable devices. These processes include:
- Device accuracy verification
- Data transmission security checks
- Regular system performance audits
- Documentation of validation procedures
Building Patient Trust
The regulatory landscape shapes patient trust through transparent data handling practices. Organizations conducting DCTs demonstrate their commitment to participant privacy by:
- Maintaining detailed records of compliance measures
- Conducting regular security assessments
- Providing clear documentation of data protection protocols
Evolving Guidelines
Regulatory bodies continue to adapt their guidelines as wearable technology evolves. The FDA’s recent guidance on digital health technologies reflects the growing need for specific frameworks addressing wearables in clinical research.
Addressing Privacy Concerns
Patient privacy is a top priority in decentralized clinical trials. Advanced encryption technologies now protect sensitive health data through multiple security layers, creating an impenetrable shield around participant information.
State-of-the-Art Encryption Methods:
- End-to-end encryption for all data transmission
- Multi-factor authentication systems
- Secure cloud storage with military-grade protection
- Real-time security monitoring and threat detection
Blockchain technology is changing the game in clinical trials by ensuring that all collected information remains unchanged and tamper-proof. This decentralized approach prevents unauthorized changes and guarantees complete transparency throughout the trial process.
Building Patient Trust Through Privacy Measures:
- Clear consent processes explaining data usage
- Regular privacy updates and notifications
- Options for participants to control data sharing
- Transparent audit trails of data access
Research teams are taking specific actions to address participant concerns:
- Having dedicated privacy officers for immediate response
- Conducting regular security assessments and updates
- Simplifying privacy policies using plain language
- Providing educational resources about data protection
These strong privacy measures create a secure environment where participants can confidently share their health data, knowing their information remains protected through every stage of the clinical trial.
Future Trends in Patient Engagement with Wearables
The world of wearable technology in clinical trials is changing quickly. New and improved biosensors are getting smaller, more precise, and able to measure complicated biological markers. These advancements make it possible to have:
- Smart contact lenses that constantly track glucose levels and pressure inside the eye
- Patch-based sensors that can be worn for a long time and are more comfortable
- AI-powered wearables that can foresee health issues before they happen
The Role of AI in Personalized Medicine
Combining artificial intelligence with wearable devices opens up new possibilities for personalized medicine. Machine learning algorithms process large amounts of patient data to:
- Determine how each individual responds to treatment
- Anticipate negative events
- Modify medication dosages on the spot
- Develop customized therapeutic interventions
Opportunities and Challenges Ahead
The future holds both exciting opportunities and significant obstacles:
Opportunities
- Integration with digital treatments
- Empowering patients through immediate health information
- Better recruitment for trials using remote monitoring features
Challenges to Overcome
- Limitations in battery life
- Need for consistent data across devices
- Integration issues with current healthcare systems
- Making costs affordable for different populations
The Next Generation of Wearables
The upcoming generation of wearables will probably use advanced materials science, introducing features such as self-charging abilities and components that break down naturally. These advancements aim to make clinical trials easier to access, more efficient, and centered around patients while providing researchers with more comprehensive data sets.
Conclusion
The integration of wearables in decentralized clinical trials marks a revolutionary shift in how we conduct medical research. These digital tools have transformed the traditional clinical trial landscape into a dynamic, patient-centric ecosystem that prioritizes engagement, accessibility, and data quality.
Key Transformative Impacts:
- Real-time health monitoring reshapes participant experiences
- Enhanced data collection leads to more accurate trial outcomes
- Improved accessibility breaks down geographical barriers
- Strengthened patient-researcher relationships drive better engagement
The future of clinical research lies in embracing these technological innovations. Organizations that adopt wearables in their DCTs position themselves at the forefront of medical advancement, benefiting from:
- Higher participant retention rates
- Reduced operational costs
- More diverse patient populations
- Better quality data collection
Now is the time for stakeholders to embrace this digital transformation. Whether you’re a research organization, healthcare provider, or technology developer, incorporating wearables into your clinical trials can create more efficient, engaging, and successful research outcomes.
Ready to transform your clinical trials? Take the first step toward enhanced patient engagement by exploring wearable integration options for your next study. Your commitment to innovation today shapes the future of medical research tomorrow.
FAQs (Frequently Asked Questions)
Decentralized clinical trials (DCTs) are innovative research methodologies that prioritize patient-centricity and allow remote participation. They leverage technology to enhance accessibility and engagement, contrasting with traditional clinical trial methods that often require in-person visits.
Wearables, such as smartwatches and biosensors, provide real-time health data and continuous monitoring of participants. This technology fosters better communication between participants and researchers, enhances the participant experience through immediate feedback loops, and ultimately increases engagement in clinical trials.
DCTs utilize a variety of technologies including health apps for data collection, telehealth platforms for remote consultations, and wearables for continuous health monitoring. These innovations facilitate participant interaction and improve trial accessibility.
The use of wearables in clinical trials can lead to increased participant diversity and engagement, cost reductions compared to traditional methods, and improved data quality due to real-time monitoring. These advantages contribute to more effective and efficient research outcomes.
Regulatory requirements such as 21CFRpart11 and GDPR are essential for ensuring compliance when using wearable technology in DCTs. These regulations help protect data security and integrity, which is crucial for maintaining patient trust throughout the research process.
Future innovations in wearable technology are expected to enhance data collection processes, supporting the growing trend towards personalized medicine. While there will be challenges to address, such as privacy concerns, the potential opportunities for improving patient engagement in DCTs are significant.