Delve Health Provides the Technology Platform to Design Clinical Trials to Fit a Patient’s Lifestyle

The Role of Clinical Assessments and Digital Tools in Measuring Mental Health Outcomes
Exploring the transformative role of clinical assessments and digital tools in measuring mental health outcomes.
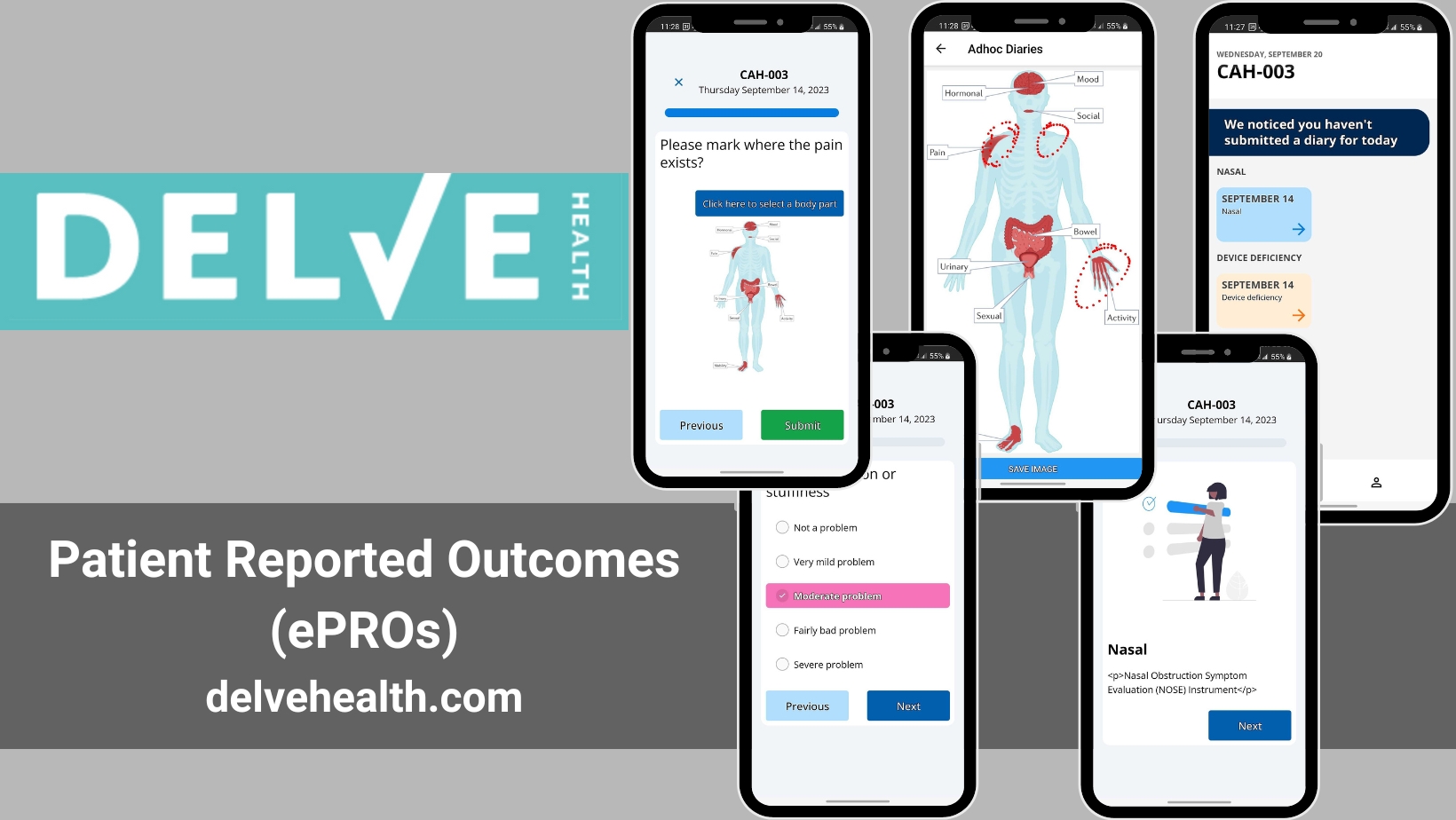

Delve Health’s Deployment of Patient Reported Outcomes in Oncology: Pathways to Success and Enhanced Patient Compliance
Introduction In the intricate field of oncology, understanding patient perspectives—how they feel and function—is crucial. Delve Health stands at the forefront of integrating Patient Reported


Case Study: Leveraging Delve Health Platform for Parkinson’s Study with Wearables Monitoring Dyskinesia
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by motor symptoms such as tremors, rigidity, and bradykinesia. Clinical trials aimed at developing treatments for PD


Navigating Wear Compliance Issues in Remote Healthcare Monitoring and Clinical Trials
In recent years, the healthcare industry has seen an exponential increase in the use of wearables for remote monitoring, both in standard healthcare settings and


Digital Endpoints and Patient Outcomes in Clinical Trials: Understanding the Differences and Similarities
In the rapidly evolving landscape of clinical trials, two terms that are gaining significant traction are “digital endpoints” and “patient outcomes.” Both play critical roles




























